Evaluation of transfusion transmissible infections surveillance system at Regional Blood Transfusion Centre Eldoret, Kenya, July 2022-June 2023
Reuben Tulel1,2,&, Fredrick Odhiambo2, Mamo Umuro1, Thomas Rotich1, 3, Hillary Barmasai1
1Kenya Blood Transfusion and Transplant Service, Nairobi, Kenya, 2Field Epidemiology and Laboratory Training Program, Nairobi, Kenya, 3Regional Blood Transfusion Centre, Eldoret, Kenya
&Corresponding author
Reuben Tulel, Kenya Blood Transfusion and Transplant Service, Nairobi, Kenya.
Introduction:
A Surveillance system for transfusion transmissible infections (TTIs) in the blood donor population is an important public health strategy for ensuring blood transfusion safety thereby disease prevention and control. Evaluating the transfusion transmissible infections surveillance system and its linkage to public health action is an important undertaking for blood transfusion programs. The Kenya National Blood Transfusion Service (KNBTS) TTIs surveillance system was evaluated to determine its usefulness in monitoring TTIs and informing blood safety strategies and linkage to public health action.
Methods:
In conducting the TTIs surveillance system evaluation, we used the CDC updated guidelines for the evaluation of public health surveillance systems. A retrospective review of blood donation data at the Regional Blood Transfusion Centre (RBTC) Eldoret was conducted between July 2022 to June 2023. Key informant interviews were conducted with officers using the surveillance system on the operations and attributes of the system. Descriptive statistics on quantitative data were performed.
Results:
There were 23,560 blood donations during the evaluation period with 16,372 (84.2%) being from males. The quality of data collected in the system was evaluated and 19,433 (82.5%) donors had complete data, while 4,127 (17.5%) had incomplete demographic and serological results data. Seropositive confirmed donations were 635 (3.3%) with TTIs seropositivity of 3.2% and 3.4% among males and females respectively. Seropositivity of TTIs by type was 1.2% hepatitis B infection, 0.9% HIV, 0.6% syphilis, and 0.5% hepatitis C. The five key officers involved in reporting into the system knew how to populate the routinely used laboratory, donor registers and monthly reporting summary tool used for reporting blood quality indicators. The data collected and key informant interviews conducted indicated the system's inability to meet its objectives with no recorded public health action following the reported rise in TTIs prevalence of 4.1% in December. The TTIs surveillance system is sensitive and can monitor trends in the number of cases over time with a total of 635 cases of TTIs detected. The surveillance system is designed to collect predefined TTIs variables and is not flexible to track other diseases if the need arises.
Conclusion:
The TTIs surveillance system at the evaluation site was found not to be meeting its objectives of informing blood safety strategies. The system is not useful as information collected does not elicit public health action to ensure blood transfusion safety. The system is acceptable and sensitive however not flexible. Strengthening utilization of data from the system to ensure blood safety and digitization will improve the general performance of the system.
Introduction

Transfusion transmissible infections (TTIs) caused by human immunodeficiency virus (HIV), hepatitis B (HBV), hepatitis C and syphilis are a global health threat, especially in Sub-Saharan Africa[1]. The World Health Organization in 2017 released its global hepatitis report in which an estimated 257 million people in 2015 were living with chronic hepatitis B infection with greater disease burden in African and Western Pacific regions. It was estimated that among 36.7 million persons living with HIV, 2.7 million had HBV infection. Hepatitis B was cited as the most prevalent blood-borne infection; despite an available effective vaccine, the fight against HBV infection is hindered by low vaccine coverage [2]. Globally its estimated that 2 billion people are infected with HBV including 400 million chronically [3]. An estimated 5-10% of HIV infections in Africa are because of unsafe blood transfusions[4]. In low- and middle-income countries (LMICs),12.5% of patients receiving blood transfusion are at risk of post-transfusion hepatitis B infection because of the high endemicity of hepatitis B infection [5].
Blood and components transfusion is an important intervention in the provision of quality healthcare services. Transfusion transmissible infections are a threat to the availability of safe blood for transfusion hence of public health concern. The World Health Organization (WHO) recommends the mandatory screening of donated blood and blood components for HIV, hepatitis B and C as well as syphilis as a key strategy of blood safety programs globally [
6]. A surveillance system for these diseases is key in planning blood safety programs including the recruitment of safe blood donors and initiating prevention and control measures[
7]. Transfusion transmissible infection surveillance systems are weak or lacking in most low and middle-income countries [
5].
The blood service ecosystem in Kenya has established standards and guidelines for the management of the blood transfusion service, however, the legal regulatory framework is undergoing strengthening through a legislative process. The service is organized in a hub and spoke model centrally coordinated with six regional blood transfusion centres and 43 satellite blood transfusion centres. The regional blood transfusion centres recruit blood donors, collect blood, process and test blood for transfusion transmissible infections and blood typing. Satellite blood transfusion centres undertake recruitment of blood donors, collect blood, process and ship blood samples for laboratory testing in the regional centres.
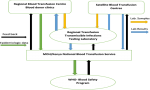
The Kenya National Blood Transfusion Service (KNBTS) TTIs surveillance system is a manual system with data captured manually from donor clinics and laboratory registers in all the blood establishments in Kenya. Donor demographic data is collected during blood donation in donor clinic registers and laboratory registers for the TTIs serology results. Monthly aggregated data is collected and submitted to the KNBTS monitoring and evaluation department for analysis and reporting on TTIs seropositivity alongside other blood safety and performance indicators (
Figure 1).
The system's objectives are to improve blood transfusion safety by informing blood safety strategies and enhancing the prevention and control of transfusion-transmissible infections in the blood donor population and community. Monthly collection of data on transfusion transmissible infections in blood donors is conducted alongside other blood safety indicators by the Kenya National Blood Transfusion Service. A surveillance system does exist that covers performance in some key indicators but has never been evaluated to determine its usefulness in ensuring blood safety and informing decision-making for public health action.
Surveillance system evaluation ensures that problems of public health importance are monitored efficiently and effectively [
8]. Blood donor screening for HIV, hepatitis B, hepatitis C and syphilis provides important data that can be used to monitor these diseases in the general population [
9]. Strong surveillance for these TTIs among blood donors provides important data contributing to blood safety, prevention, and control measures of these diseases in the communities [
10].
We assessed the TTIs surveillance system at the Regional Blood Transfusion Centre Eldoret to determine its usefulness, the system attributes and whether the system meets its objectives. This will help understand TTIs among the blood donor population at the evaluation site and inform blood donation activities to achieve blood safety and public health action.
Methods


Study setting
The Regional Blood Transfusion Centre Eldoret in the North Rift Region is one of the six regional blood banks in Kenya. The centre receives blood donations from eligible blood donors in the region through walk-in donations and outreach activities. Laboratory processing of blood including blood components preparation and laboratory testing is undertaken at the centre. The Regional Testing Laboratory undertakes donor blood sample screening for HIV, Hepatitis B, Hepatitis C, Syphilis and ABO blood typing. The centre provides TTIs testing services for County Satellite Blood Banks in Trans-Zoia, West Pokot, Bungoma, Nandi, Elgeyo Marakwet and Turkana counties mapped to the region.
Evaluation design
We conducted a descriptive cross-sectional study through a retrospective review and analysis of blood donor data at Regional Blood Transfusion Centre Eldoret with a focus on the descriptive analysis and surveillance system attributes between July 2022 to June 2023. The updated CDC guidelines for the evaluation of public health surveillance systems were used in the evaluation.
Case definition
Cases in the TTIs surveillance system are donations with laboratory seropositive results for HIV, Hepatitis B, Hepatitis C or Syphilis and confirmed as per the KNBTTS testing algorithm adopted from the WHO recommendations on screening of donated blood for transfusion transmissible infections [
11].
Data collection
Data on all blood donors who donated blood at the regional blood transfusion centre in Eldoret between July 2022 to June 2023 was abstracted from the donor clinic and laboratory registers. All demographic and laboratory data on blood donors who donated blood in the period were reviewed, and variables on age, gender, HIV, Hepatitis B, Hepatitis C and Syphilis serological results were entered into a Microsoft Excel Office, 2013 spreadsheet for analysis. Aggregated monthly data reported to the KNBTS monitoring, and evaluation unit was also used for comparison. A total of nine officers with reporting responsibilities were interviewed using a structured questionnaire at the study site among them were six laboratory officers responsible for laboratory testing of blood for TTIs, one health records and information officer responsible for monthly summary report compilation and two nurses responsible for pre and post-donation counselling.
Data variable extraction
Demographic data variables on age, gender and serology laboratory results on HIV, Hepatitis B, Hepatitis C and Syphilis were extracted from the donor clinic and laboratory registers to achieve the objectives of the transfusion transmissible infections surveillance system evaluation.
Evaluation of the system's usefulness and attributes
Review of data and information collected in the key informant interviews to establish the contribution of the system in the detection of changes in trends of transfusion transmissible infections among blood donors and its ability to initiate public health action in prevention and control and if it is meeting its objectives.
Simplicity- This was described as the ease of operation of the TTIs surveillance system structure and ease of ascertaining cases. This was assessed by looking at the variables captured by the transfusion transmissible infections surveillance system and how reporting flows in the system (
Table 1).
Flexibility- This was based on the ability of the TTIs surveillance system to be integrated or broadened to be used for surveillance of other diseases (
Table 1).
Sensitivity- This is the ability of the system to detect cases and disease trends. The TTIs surveillance system was evaluated on its ability to track cases over time thereby giving trends.
Data Quality- This was described as the accuracy, completeness and timeliness of the data captured by the transfusion transmissible infections surveillance system. The data collected in the laboratory clinic and laboratory registers were checked for completeness of variables on serological results, age, and gender (
Table 1).
Acceptability- This was described as the willingness of the responsible officers to report in the TTIs surveillance system. This was measured through key informant interviews and a review of donor forms for completeness.
Representativeness- This was described as the ability of the TTIs surveillance system to describe the occurrence of TTIs over time and its distribution in population by place and person.
Data management
The data was collected then cleaned and donations without key laboratory and demographic data were reported and removed from the evaluation. The results from the laboratory registers were confirmed as per the KNBTTS-approved testing algorithm where reactive test results for HIV, Hepatitis B, Hepatitis C or Syphilis are confirmed before reporting into the registers. Information on the system´s attributes and usefulness was collected using data review and a structured questionnaire used in the key informant interviews.
Data analysis
We analyzed collected data quantitatively and qualitatively with direct content analysis for qualitative data. As guided by the CDC guidelines for the evaluation of public health surveillance systems the system attributes were selected as themes for analysis.
We conducted a descriptive analysis of the abstracted data using Microsoft Excel Office 2013 and data was organized in tables and figures. Proportions were calculated for selected variables on gender, age groups and laboratory serological data.
The data from the key informant interviews for the system attributes were analysed to get the proportions of the responses. The data was then presented in tables and graphs.
Ethical consideration
The Kenya Field Epidemiology and Laboratory Training Program (KFELTP) and Moi University School of Public Health approved the protocol. The surveillance system evaluation involved the collection and analysis of routine data and information hence no ethical review was sought. Permission to use blood donor data was sought and granted by the Kenya National Blood Transfusion Service. To ensure the confidentiality of the donors, the data used was anonymized by omitting donor personal identifiers and donation numbers.
Results


Characteristics of blood donors and key informant interview respondents
During the evaluation period, a total of 23,560 persons donated blood at the Regional Blood Transfusion Centre, Eldoret. Of these, only 19,433 (82.5%) had all the required information. Among these 16,372 (84.2%) were males, Blood donor ages ranged between 16 to 65. Most blood donors were aged between 18 to 25 with 6,774 blood donors representing 34.9%, while the least were aged 16 to 17 with 237 blood donors representing 1.2% (
Table 2). Most of the respondents were males (5/9) and all had extensive experience in the blood transfusion service.
Assessment of the usefulness of the system
From the data reviewed, the TTIs surveillance system tracks the trend in the prevalence of HIV, Hepatitis B, Hepatitis C, and syphilis over time. Seropositive donations are confirmed using the KNBTTS testing algorithm. The system meets its reporting requirements as monthly summary reports are submitted to the KBTTS monitoring and evaluation unit, and no analysis is done at the regional level. The information collected from the key informant interviews and data review did not reveal any public health action instituted nor change in strategy in blood safety program at the study site or national level. For the blood donor notification, there was no clear referral system for donors with seropositive donations for care and treatment.
Data Quality: In the data review, 19,433 (82.5%) donations had complete demographic and laboratory serology variables, and 4,127 (17.5%) had incomplete demographic and laboratory variable data such as age, gender, or confirmation results.
Sensitivity: The Transfusion Transmissible Infections surveillance system can monitor cases over time, therefore, trends in the prevalence of transfusion transmissible infections. In the evaluation period, the ability of the system to track trend of seropositivity by month and type of TTI was evaluated. Seroprevalence was highest in December at 4.1% and lowest in November at 1.8% (
Figure 2). Three per cent (635/19,433) of the blood donors had seropositive TTIs results. TTI seropositivity was 3.2% (531/16,372) among male and 3.4% (104/3061) among female donors (
Table 3). Seropositivity by type of TTI was HIV 0.9%, HBV 1.2%, HCV 0.5%, and Syphilis 0.6% (
Figure 3).
Simplicity: Through the administered questionnaire on simplicity, six out of nine respondents (67%) indicated that the system structure was simple despite challenges in the retrieval of data on serological results for notification of blood donors. The five key officers involved in reporting into the system knew how to populate the routinely used laboratory, donor registers and monthly reporting summary tool used for reporting blood quality indicators. The respondents also indicated a clear and simple identification of cases through the confirmed TTIs serology results captured in the laboratory register and utilized in the system.
Flexibility:The TTIs surveillance system data variables collected are predefined for the TTIs making the system rigid for new data requests to track other diseases.
Acceptability: The completeness of data in the system was 82.5% (19,433/23,560) and 87.5% (7/8) of the key informants indicating their willingness to report into the system.
Discussion


The evaluation of public health surveillance systems such as the TTIs surveillance system is to ensure that problems of public health importance are monitored efficiently and effectively [12]. Robust surveillance systems in the blood transfusion service are important to support the safety of the blood supply and are of high priority because of public expectation of zero risk in transfusion[13]. The findings from the evaluation showed that the system utilizes standard tools and users of the system were knowledgeable about the operations of the system and the blood transfusion service in general. However, the system was found not to be useful and therefore did not meet its objectives of informing decision-making on the safety of blood supply and TTIs prevention and control strategies. This disagrees with an infectious disease surveillance publication and the goals of surveillance going against its objective of informing blood safety at the evaluation site [14]. Lack of a clear notification and referral of seropositive blood donors for care and treatment impedes control and prevention of HIV, hepatitis B, C and syphilis in the region. The incompleteness of data on blood donors' key variables could be attributed to challenges in donor data capture during donor registration in blood drives. This affects the quality of data in the system and is comparable to the findings in the evaluation of the animal rabies surveillance system in Ghana in which reports data was inconsistent [15]. The TTIs surveillance system is sensitive, tracking cases by person, place and time, this is an important attribute as it can be used in the planning of blood donation activities to target low-risk populations hence safe blood supply. Regarding simplicity, the TTIs surveillance system is simple in structure and tools with clear identification of cases however the manual nature of the system makes retrieval of blood donor information difficult. This negatively impacts the notification of blood donors on their serology results hence missing the opportunity for linkage to care and treatment for seropositive donors. The system is not flexible and therefore cannot adapt to changing information needs other than HIV, Hepatitis B, Hepatitis C or Syphilis surveillance, however, the residual serum samples can be used for surveillance of other blood-borne diseases.
The performance of the surveillance system can be improved by the digitalization of the blood transfusion processes for quality data capture. The scope of the evaluation was however conducted within a limited time at the Regional Blood Transfusion Centre Eldoret representing one out of the six sites of the Kenya National Blood Transfusion Service therefore may not reflect the performance of the system in other regions of Kenya. The evaluation was not also exhaustive as other attributes such as stability, timeliness and positive predictive value were not considered in the evaluation.
Conclusion


The TTIs surveillance system is of public health importance in ensuring blood transfusion safety. A robust and effective surveillance system in blood transfusion service in addition to ensuring blood safety also allows for case detection through screening of HIV, Hepatitis B, C and syphilis in communities bolstering control and prevention strategies. However, the TTIs surveillance system is only used for routine reporting without data utilization and follow-up public health action and hence does not meet its usefulness in informing blood safety and disease control and prevention. The TTIs surveillance is sensitive and simple, however, the lack of completeness of data collected for some blood donors affects the surveillance system quality. The manual nature of the system makes it difficult to retrieve data, especially serological results for post-donation counselling and referral to care and treatment of seropositive blood donors. Measures to modify and strengthen the system are vital to achieve blood safety by Kenya National Blood Transfusion Service.
Recommendations
Considering the key public health data and information captured during blood donation and the potential for use in the control and prevention of TTIs, we recommend that the Kenya National Blood Transfusion Service digitizes the blood transfusion services. This will improve the quality and retrieval of data reported in the surveillance of transfusion transmissible infections and inform blood safety and linkage to care and treatment for seropositive blood donors. There is a need for KNBTS to establish a feedback mechanism on the reports generated from the data collected to inform decision-making by the blood establishments in the country. To further improve blood transfusion safety and disease prevention and control in the country, leveraging on the blood donor population in the surveillance of other TTIs is important considering their epidemiology in the country.
Public health action
The findings of the evaluation were presented to the staff at the Regional Blood Transfusion Centre Eldoret with emphasis on the accuracy and completeness of data captured in the donor clinic and laboratory registers. There is a need to review the data captured and reports generated from the TTIs surveillance system to inform blood safety. Emphasis on the importance of the blood donor population as key in the early control and prevention of diseases through surveillance. Blood donor notification and linkage to care and treatment, especially for seropositive donors, was emphasized and identified as a strategy for the prevention and control of TTIs.
Competing interests


The authors declare no competing of interests.
Authors´ contributions


RT drafted the evaluation protocol, RT, and TR, HB participated in the data collection, and RT drafted the manuscript, FO reviewed the manuscript.
Acknowledgements


Sincere appreciation goes to the following individuals and institutions for their various contributions and roles played in my resident training:
Mr. Mamo Umuro, Head of National Blood Transfusion Service, Mr. Thomas Rotich, Regional Manager, Eldoret and Mr. Fred Odhiambo, Faculty, Kenya Field Epidemiology and Laboratory Training Program.
Tables and figures


Table 1: Sociodemographic characteristics of blood donors
Table 2: Seroprevalence of Transfusion Transmissible Infections
Table 3: Seroprevalence of TTIs among blood donors in RBTC-Eldoret July 2022 - June 2023
Figure 1: Data Flow
Figure 2: Trend of TTIs seroprevalence
Figure 3: TTIs seroprevalence by type
References


- Peliganga LB, Mello VM, De Sousa PSF, Horta MAP, Soares ÁD, Nunes JPDS, Nobrega M, Lewis-Ximenez LL.Transfusion Transmissible Infections in Blood Donors in the Province of Bié, Angola, during a 15-Year Follow-Up, Imply the Need for Pathogen Reduction Technologies. Pathogens [Internet]. 2021 Dec 17 [cited 2024 May 1];10(12):1633. https://doi.org/10.3390/pathogens10121633 Google Scholar
- World Health Organization. Global Hepatitis Report, 2017 [Internet]. Geneva (Switzerland): World Health Organization; 2017 Apr 19 [cited 2024 May 1]. 68 p. Download 9789241565455-eng.pdf.
- Allain JP, Owusu-Ofori S, Ye X, Bisseye C, Chaar M, Li C. Hepatitis B Virus Chronic Infection in Blood Donors from Asian and African High or Medium Prevalence Areas: Comparison According to Sex. Viruses [Internet]. 2022 Mar 24 [cited 2024 May 1];14(4):67. https://doi.org/10.3390/v14040673 PubMed | Google Scholar
- Fessehaye N, Naik D, Fessehaye T. Transfusion transmitted infections - a retrospective analysis from the National Blood Transfusion Service in Eritrea . Pan Afr Med J [Internet]. 2011 Aug 18 [cited 2024 May 1];9:40. https://doi.org/10.4314/pamj.v9i1.71219 PubMed | Google Scholar
- Aliyo A, Ashenafi G, Adem S.Evaluation of Transfusion Transmissible Infections Prevalence and Trend Among Blood Donors Attended at Bule Hora Blood Bank, West Guji, South Ethiopia. Health Serv Res Manag Epidemiol [Internet]. 2022 Nov 3 [cited 2024 May 1];9:233339282211367. https://doi.org/10.1177/23333928221136717 PubMed | Google Scholar
- Kimani D, Mwangi J, Mwangi M, Bunnell R, Kellogg TA, Oluoch T, Gichangi A, Kaiser R, Mugo N, Odongo T, Oduor M, Marum L, for the KAIS Study Group. Blood donors in Kenya: a comparison of voluntary and family replacement donors based on a population–based survey . Vox Sang [Internet]. 2010 Aug 25 [cited 2024 May 1];100(2):212-8. https://doi.org/10.1111/j.1423-0410.2010.01376.x Subscription or purchase required to view full article. Google Scholar
- World Health Organization. Training workshop on screening, diagnosis and treatment of hepatitis B and C [Internet]. Geneva (Switzerland): Word Health Organization; 2020 Jul 28. Module 11-Clinical management of hepatitis B virus infection: case studies; [cited 2024 May 1]. p.156-171. Download pdf to view full document.
- Calba C, Goutard FL, Hoinville L, Hendrikx P, Lindberg A, Saegerman C, Peyre M. Surveillance systems evaluation: a systematic review of the existing approaches. BMC Public Health [Internet]. 2015 May 1[cited 2024 May 1];15(1):44 https://doi.org/10.1186/s12889-015-1791-5 PubMed | Google Scholar
- Ly KN, Kim AA, Umuro M, Drobenuic J, Williamson JM, Montgomery JM, Fields BS, Teshale EH.Prevalence of Hepatitis B Virus Infection in Kenya, 2007 . Am J Trop Med Hyg [Internet]. 2016 Aug 3 [cited 2024 May 1];95(2):348-53. https://doi.org/10.4269/ajtmh.16-0059 Subscription or purchase required to view full article. PubMed | Google Scholar
- Wamamba D, Onyango D, Oyugi E, Kanyina E, Obonyo M, Githuku J, Ransom J.Transfusion Transmissible Infections Among Walk-In Blood Donors at Kisumu Regional Blood Transfusion Centre, Kisumu County, Kenya, 2015 . Lab Med [Internet]. 2017 Sep 23 [cited 2024 May 1];48(4):362-6. https://doi.org/1093/labmed/lmx059 Google Scholar
- World Health Organization. Screening Donated Blood for Transfusion-Transmissible Infections: Recommendations [Internet]. Geneva (Switzerland): World Health Organization; 2009 Jan 1 [cited 2024 May 1]. 66 p. Download pdf to view full document. Google Scholar
- German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN, Guidelines Working Group Centers for Disease Control and Prevention (CDC). Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group . MMWR Recomm Rep [Internet]. 2001 Jul 27[cited 2024 May 1];50(RR-13):1-35; quiz CE1-7. Google Scholar
- O´Brien SF, Zou S, Laperche S, Brant LJ, Seed CR, Kleinman SH.Surveillance of transfusion-transmissible infections comparison of systems in five developed countries . Transfus Med Rev [Internet]. 2011 Sep 25 [cited 2024 May 1];26(1):38-57. https://doi.org/10.1016/j.tmrv.2011.07.001 PubMed | Google Scholar
- Murray J, Cohen AL.Infectious Disease Surveillance . In: International Encyclopedia of Public Health [Internet]. 2nd ed. Amsterdam (Netherlands):Elsevier; 2016 Oct 24 [cited 2024 May 1]. p. 222-9. https://doi.org/10.1016/B978-0-12-803678-5.00517-8 PubMed | Google Scholar
- Guri BZ, Acquah H, Bandoh DA, Noora CL, Kaburi BB, Denueme S, Khumalo GK, Afari E A, Kenu E.Evaluation of animal rabies surveillance system, Sunyani West District- Ghana, 2019. JIEPH [Internet]. 2023 Sep 18 [cited 2024 May 1];6(3):14. https://www.doi.org/10.37432/jieph.2023.6.3.86 Google Scholar