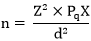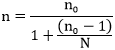Occurrence and risk of human infection and antimicrobial susceptibilities of escherichia coli isolates from beef sold for human consumption in Jos metropolis, Plateau state Nigeria, September 2018 - June 2019
Yohanna Iliya1,&, Jacob Kwada Paghi Kwaga2, Grace Sabo Kia2, Yakubu Gunya Dogonyaro Dashe3, Godwin Ojonugwa Agada3, Muhammad Shakir Balogun1, Charles Michael Akatobi1
1Nigerian Field Epidemiology and Laboratory Training Programme Asokoro, Abuja, Nigeria, 2Department of Public Health and Preventive Medicine, Ahmadu Bello University, Zaria, Kaduna State, Nigeria, 3Central Diagnostic Laboratory, National Veterinary Research Institute, Vom, Jos, Plateau State
&Corresponding author
Yohanna Iliya, Nigerian Field Epidemiology and Laboratory Training Programme Asokoro, Abuja, Nigeria. yohannailiya95@yahoo.com
Introduction:
Antimicrobial resistance poses a threat to infection management globally. We determined the prevalence and antimicrobial susceptibilities of Escherichia coli (E. coli) isolates from beef and assessed beef seller's practices and awareness of antimicrobial resistance (AMR) from Jos metropolis, Plateau State, Nigeria.
Methods:
We recruited 114 beef sellers and collected beef samples for the study. Information on socio-demographic characteristics, practices and awareness on AMR were collected using a semi-structured interviewer-administered questionnaire. The isolates were tested for susceptibilities to 14 antibiotics using Kirby-Bauer disk diffusion method and the Minimum Inhibitory Concentration (MIC) of Ciprofloxacin using commercially prepared evaluator strips (Oxoid, UK).
Results:
The mean age of the respondents was 35 ±12.5 years. All were males among whom 90 (78.9%) had post primary education. There were poor (110/114) beef handling practices among all age groups as well as poor awareness of AMR 110 (96.4%) among the respondents. Age, education and duration in the business had no significant impact on beef seller's handling practices and awareness on AMR (P > 0.05). Thirty E. coli isolates (26.3%) were obtained of which 29 (92.8%) exhibited Multi Drug Resistance (MDR) with 4 (13.3%) isolates being positive for extended spectrum beta lactamase production. More than 90% of the isolates had multiple antibiotic resistance index of greater than 0.2. Twenty-two (75.9%) of the isolates were resistant to ciprofloxacin by MIC method.
Conclusion:
Beef handling practices was found to be inadequate to render beef wholesome for human consumption in Jos South LGA, Plateau State. Escherichia coli was prevalent on fresh beef and the E. coli isolates exhibited multidrug resistance phenotype. Most antibiotics tested in this study may no longer be very effective (> 50% resistance) for treatment of E. coli infection in humans. We recommend to the Plateau State government through the Ministry of Agriculture to institute policies that will increase surveillance on zoonotic bacterial agents and antibiotic susceptibilities in food of animal origin. Also, the State should build/organise beef markets and institute proper hygiene in abattoir facilities and for beef handlers.
Introduction

Escherichia coli (E. coli) are commensal bacteria in the intestine of cattle as well as humans [1,2]. Consumption of food or water contaminated with pathogenic E. coli can cause diarrhoea [3]. It can also cause pneumonia, respiratory problems and urinary tract infection [4]. Other strains of the organism produce toxins that damage the lining of the intestine, while strains like E. coli 0157:H7 cause severe illness that result in muscle cramp, abdominal pain, bloody diarrhoea and acute kidney failure in children. Some of the pathogenic E. coli strains may produce Shiga-like toxins that may cause severe illness and are members of a class of pathogenic group of E. coli known as enterohaemorrhagic E. coli (EHEC). They can also be classified by their toxin producing capabilities to include; verocytotoxin-producing E. coli (VTEC) or Shiga-like toxin producing E. coli (STEC) [3,5]. E. coli of animal origin may act as donors of antimicrobial resistance genes for other pathogenic E. coli. Thus, the intensive use of antimicrobial agents in food animals may add to the burden of antimicrobial resistance in the human population [6].
Globally, food borne illness due to
E. coli is a growing concern today. It is estimated that 600 million people fall sick after consuming food contaminated with food-borne pathogens worldwide with 420,000 deaths including 125,000 children under the age of 5 years according to WHO [
7]. Africa and South-East Asia regions were more affected. In Africa, outbreaks of Shiga-toxin-producing
E. coli were reported in all parts of the continent [
5,
8] and isolation of entero-haemorrhagic
E. coli 0157 have also been documented in humans and animals. Despite advances in food technology, foods of animal origin were still found to be contaminated with
E. coli. In Ethiopia, susceptibility profiles of
E. coli was reported to have substantial geographic variations as well as significant differences in various populations and environments [
9]. In Nigeria, the prevalence of
E. coli contamination of beef and beef product were reported in Kaduna, Kano and some states in the south west and south east [
10,
11].
Bacterial contamination of food of animal origin pose public health risks to humans, either through contact with contaminated food of animal origin or through cross-contamination with other food or through contamination with pathogenic bacteria.
E. coli has been identified to be an indicator organism for the occurrence and spread of pathogens that can be transmitted through food-borne infections to humans. It is also an indicator for faecal contamination hence, the occurrence of
E. coli indicates the likelihood of contamination with other food-borne pathogens of faecal origin [
1]. It is also indicator for the spread of antimicrobial resistance (AMR) genes in the environment. Also,
E. coli of animal origin may act as donors of antimicrobial resistance genes for other pathogenic
E. coli and other bacteria [
6]. Acquired antibiotic resistance among bacterial organisms is a growing challenge globally, that is made worse by overuse of antibiotics in humans and food animals [
11]. Bacteria from the animal reservoirs that show resistance to antimicrobial agents that are regarded as highly or critically important in human therapy (e.g. aminoglycosides, fluoroquinolones, and third- and fourth-generation cephalosporin) [
12] are of great concern in the world today as well as in Nigeria. Currently, there is limited information available on the occurrence and antimicrobial susceptibilities of
E. coli in Jos South LGA of Plateau State.
Therefore, we investigated practice of beef sellers and their awareness on AMR, occurrence and antimicrobial susceptibilities of selected antimicrobials and the minimum inhibitory concentration (MIC) of Ciprofloxacin for resistance
E. coli isolates from beef in Jos South LGA of Plateau state, Nigeria.
Methods


Description of study area
The study was conducted in Jos South Local Government Area of Plateau State located in the North central zone of Nigeria
Figure 1. Jos South is one of the metropolitan LGAs of the state with its headquarters located in Bukuru town on 9°48´00” N 8°52´00” E. It occupies an area of 510 km
2 and a population of 306, 716 based on 2006 census. The major indigenous tribe is Berom, but English is the official language, although Hausa has gained acceptability as a medium of communication. The major occupation of the inhabitants is predominantly farming, tin mining, business and some few civil servants. Jos South LGA has five districts, 20 wards, and three abattoirs. The city is located on the Jos Plateau at an elevation of about 1,238 meters above sea level.
Study design, study population and sample size determination
The study adopted a cross sectional study design. The study population comprised of beef sellers within abattoirs and retail markets in Jos South LGA. Beef samples were collected from each of the sellers. All fresh beef sold at retail points of sales in abattoirs and wards retail markets in Jos South LGA were included in the study, while all beef kept overnight or those which showed sign of spoilage were excluded. A total of 114 fresh beef samples were obtained and 114 questionnaires were administered concurrently to beef sellers to carry out the study. The sample size was calculated using the method and formula described in a study conducted on sample size calculation by Pourhoseingholi et al., (2013) [
13].
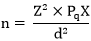
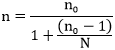
(population finite correction) x 1/(1-f)
Where - n = is the minimum sample size
Z = is the statistic corresponding to level of confidence at 95%
p =expected prevalence
q=1-p
d = level of precision will be taken at 5%
no = calculated sample size
N = total population
F = non response rate
Sampling technique
Multistage sampling technique was used for selection of the study respondents. Jos Metropolis is composed of three LGAs. Stage 1: Selection of LGA: The 3 LGAs in Jos metropolis were listed and selection of one LGA was done by balloting. Jos South LGA was the one selected. Stage 2: Selection of wards: All the wards in Jos South LGA were listed. Two wards were selected from each of the district using simple random sampling. Stage 3: Selection of beef sellers from each selected wards was done based on purposive sampling for each ward.
Data Collection and analysis (questionnaire)
Pre-tested semi-structured interviewer-administered questionnaire was used by trained data collectors to obtain data on beef sellers´ socio demography, practices, and their awareness on AMR. Beef samples from abattoir retail sellers and ward retail point of sales were collected from every beef seller until the required sample size was obtained (butcher pair). About 25g of beef samples were obtained from retail beef sellers aseptically into polythene bags that were placed on cold ice packs in a Coleman flask. These were transported from the points of sample collection to the Central Diagnostic Laboratory at the National Veterinary Research Institute (NVRI), Vom, for processing and processed immediately or kept in the refrigerator at 4°C and processed within 12 hours. Data generated from the questionnaires were analyzed using Epi info version 7 and Microsoft Excel 2007. Univariate analysis was carried out to determine the frequencies and measure of central tendency for the different variables. Chi-square analysis was used to determine the association between the independent variables (age of beef sellers´, education level and duration in the business) and dependent variables (beef sellers´ practices and the awareness on AMR) at 0.05 level of significance. The practices and the awareness were scored using median score (35) for awareness and 50% for beef sellers´ practices to categorize them into “poor” or “good” respectively.
Laboratory investigation
Isolation and identification of E. coli
Isolation and identification of
E. coli was done using conventional test methods (indole, TSI, citrate, urease, Voges-Proskauer and Methyl red) and the commercially prepared kit (Microbact 24E (Oxoid) to purify the organism. The Micrbact reading was done by comparing with the Microbact 24E interpretation guide (colour chart) and the octal coding system was adopted for Microbact 24E, where each group of three Microbact chemical reactions produced a single digit of the code. These codes were inputted into the computer software package for the interpretation of possible organism carried out and interpreted based on the recommendations of the manufacturer (Oxoid, Basingstoke, UK).
Antibiotic susceptibility test of E. coli isolates
Susceptibility tests for the
E. coli isolates was done using Kirby Bauer disc diffusion method [14]. Fourteen antibiotics namely; (Amoxicillin-Clavulanic acid (AMC)30 µg, Sulphamethoxazole-Trimethoprim (SXT)19:1(SXT) 25 µg, Ciprofloxacin (CIP) 5 µg, Chloramphenicol (C) 10 µg, Cephazolin (KZ) 30 µg, Ceftriaxone (CRO) 30 µg, Cefuroxime Sodium(CXM) 30 µ g, Cefepime (FEP) 30 µg, Gentamicin (CN) 10 µg, Tetracycline (TE) 30 µg, Meropenem (M) 10 µg, Erythromycin (E) 15 µg, Colistin (CT) 10 µg and Nalidixic acid (NA) 30 µg Oxoids) were used to carry out the tests. The interpretation of zones of inhibition was done according to Clinical and Laboratory Standards Institute (CLSI) 2018.
Escherichia coli ATCC 43888 was used as quality control organism for the antibiotic disc. In addition, for true test of resistance for
E. coli, Extended Spectrum Beta-Lactamase (ESBL) test was carried out using four antibiotic disc (Amoxicilin-clavulanate (AMC), Ceftazidime (CAZ), Ceftriazone (CRO) and Cefepime (FEP). The AMC was used as beta lactamase inhibitor. The ESBL production was inferred when the zone of inhibition around Ceftazidime and other disc were expanded by the clavulanate [
15].
Minimum inhibitory concentration Test
Minimum Inhibitory Concentration (MIC) was conducted for Ciprofloxacin using commercially prepared evaluator strips (Oxoid, UK) and was interpreted according to CLSI, 2015 interpretive criteria used for decrease susceptibility testing of
Salmonella and
E. coli. This was done as it is considered an important tool for confirming resistance to a given antimicrobial agent [
16].
Ethical considerations
Approval was obtained from the Research Ethical Committee of Plateau State Ministry of Health with reference number PSSH/ADM/ETH. Co/2019/005 and permission obtained from Plateau State Ministry of Agriculture. Informed consents of the participants were obtained and they were assured of their confidentiality of the information supplied. The study was harmless.
Results


Demographic characteristics of beef sellers
A total of 114 beef sellers participated in this study with one beef sample obtained per beef seller. Those aged 20-29 (29.8%) years accounted for the highest proportion. The mean age was 35 ± 12.5 years. All were males and 90 (78.9%) had post primary education. The years of experience in the business varied from 1-75 with the majority 76(66.7%) having <20 years´ experience. Beef handling practices was poor among all the age groups especially among those that had primary education 24 (100.0%). Duration in the business seemed to have little or no impact on beef handling practices among them
Table 1.
However, this association was not significant (X
2 = 2.8561, 1.2553, 1.5405 P>0.05). There was poor awareness on AMR with 110 (96.4%) among the beef sellers being unaware. Unawareness was 100% among age groups 30-39 and 50-59 years and those that had primary education. Age (P-value = 0.7222, education level (P-value = 0.5339) and duration in the business (P-value = 0.8194) had no significant impact on beef seller´s beef handling practices and awareness on AMR (Age P-value = 0.0679, educational level P-value = 0.5523 and duration in the business = 0.8333) (X
2 = 10.2723, 1.1875, 1.4620, P>0.05)
Table 2.
Most of the beef sellers 112 (98.25%) experienced sickness within a range of 1-12 times per year. Of these, only 59 (51.8%) indicated that they went to the hospital whenever they were sick highlighting the preponderance of self-medication which could promote AMR.
Occurrence and antibiotic susceptibilities of E. coli isolates from beef
Out of the 114 beef samples collected, 30 isolates were confirmed to be
E. coli Table 3 giving a prevalence of 26.3%. Susceptibilities of
E. coli isolates showed that 29 (92.8%) out of the 30 isolates exhibited multi antibiotic resistance (MAR) to various antibiotics
Figure 2 and
Figure 3 with more than 90% of the isolates having multiple Antibiotic resistance index of > 0.2. However, only 4 (13.3%) were positive when subjected for extended spectrum beta lactamase test.
Minimum inhibitory concentration of ciprofloxacin
Minimum Inhibitory Concentration test was done for ciprofloxacin for the 29
E. coli isolates which were not susceptible to ciprofloxacin by disk diffusion. Of these, 22 (75.9%) of the isolates were confirmed to be resistant to ciprofloxacin by MIC method.
Discussion


The bacterial load found in beef sold for human consumption as seen in this study may be attributed to the unhygienic practices by the beef vendors from the point of processing to the point of sale. This predisposes the consumer to food-borne infection and intoxication [17]. Good hygiene practices remains the key in promoting public health as well as beef business in developing countries [18] including Nigeria.. Our findings disclosed that males dominated beef business in Jos South LGA, unlike other food processing business activities [19]. From this study, most of the beef sellers were found to have poor beef handling practices, which is in agreement with the findings of a study conducted in Turkey [20] and in the USA which found unsafe food hygiene practices in men than the women [21].
The study also demonstrates that those who attended secondary 72(63.2%) or tertiary 18(15.7%) education have significantly better beef handling practices compared to those that attended primary education 24 (21.1%), This may probably be due to the educational awareness exposure, which is consistent with the findings of the study done in South Western Nigeria [
19] and in Iran [
22]. Our study also, revealed that individuals with fewer years of working experience as beef sellers had good practices of safe beef handling, which is not consistent with the findings of a study conducted in Benin, Nigeria which found that food handlers with longer years of experience had better practice of food hygiene and safety [
17].
However, our findings suggest that, there appears to be an increase in attitudinal negligence of hygiene practices by the beef sellers as their years of experience increased. This is consistent with the findings of a study conducted in South Western Nigeria, which found that meat handlers with lower years of working experience had significantly higher knowledge, attitude and practice levels of safe meat handling [
19].
There was poor awareness of AMR among beef sellers. Antimicrobial resistance appears to be a phenomenon which is not known to many people. This finding is similar to the recent national survey on AMR conducted in Nigeria in 2017, a review on AMR [
23] and a national survey conducted in Japan on public knowledge and perception about antimicrobials and AMR [
24].
Raising awareness on AMR among beef sellers should be an urgent priority for public and animal health programs in Plateau State specifically and Nigeria in general, as part of the call for action as recommended by WHO [
25]. This is because beef sellers are one of the groups that may contribute to the rapid spread of AMR in the course of their business. This is made more probable by beef sellers´ attestation of falling sick (98.25%) 1-12 times in a year with 58.8% illness attributed to malaria and typhoid fever with many adopting self-medication. This could be a contributor to outbreaks of MDR typhoid fever due to
Salmonella enterica reported recently by WHO in Pakistan, across Asia and Africa and could also contribute to malaria resistance [
26,
27].
Creating public awareness on AMR through communication, education and training is essential to reduce the menace of AMR in public health [
25]. However, this is contrary to the findings of a study conducted on effects of health communication which found that global campaign on AMR may not work, rather it will be counterproductive [
28].
Our study revealed the contamination of beef with
E. coli at retail points of sale in the study area was prevalent, which is consistent with the findings of the study conducted in Côte d´Ivoire [
29] and the report in the Washington post 2019. Prevalence of
E. coli on fresh beef at point of sales may pose a significant public health risk [
30,
31] which may possibly account for a substantial degree of morbidity and mortality in adult and children [
32]. The effectiveness of treatment with drugs indicated for
E. coli infections may be hampered by AMR that may have been developed by the organism [
33].
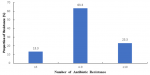
The study also revealed that, most 29 (92.8%) of the isolates exhibited multi drug resistance to most commonly used antibiotics with more than 50% resistance rate to the antibiotics tested
Figure 3, which is consistent with the findings of a study conducted in Spain and India [
34,
35] respectively, where the resistance of test organisms to similar drugs were found to be >50%. Resistance to gentamicin and cefepime were 50%, which is similar to the findings of a study conducted in Brazil and North East Ethiopia [
9,
36] respectively. Whereas resistance to sulphamethoxazole-trimothoprim were 43.3% which is lower than the findings of the study conducted in Uganda [
37] but higher than the findings of a study conducted in Australia [
38]. Fifty to 70% of the isolates were resistant to cefuroxime (CXM), amoxycilin-clavucilin and chloramphenicol. The highest rate of susceptibility to antimicrobials by the isolates was to colistin (90.0%) while MAR was found in 96.7% of the isolates. The overall low levels of non-susceptibility of the isolates were to ciprofloxacin that is indicated for the treatment of
E. coli enteric infections [
37].
This study also, determined the MIC value of ciprofloxacin for
E. coli isolates that were found to be resistant to ciprofloxacin. The lowest MIC breakpoint value of <0.12 µg/ml was used [
39] which is in line with the current CLSI, (2015) guidelines for decreased susceptibility to ciprofloxacin which states that MIC≥0.12 µg/ml should be used as a marker for the emerging fluoroquinolone resistance [
39]. This was done to determine the lowest concentration of ciprofloxacin that can inhibit the growth of
E. coli organisms. Greater percentage (75.9%) of the isolates was found to have resistant to Ciprofloxacin by MIC method. This poses a potential threat to public health as most of the isolates showed resistance to ciprofloxacin which is one of the drug of choice for treatment of
E. coli infection in humans. This study revealed that the efficacy of the drug on
E. coli is diminishing due to resistance which allow the organism to thrive.
Limitations
Due to financial constraint, only three (ciprofloxacin, AMC and ceftriaxone) out of the 14 antibiotics was selected for MIC. Consequently, due to unavailability of Amoxycilin-Clavulanate and ceftriaxone evaluator strips, only ciprofloxacin that was obtained was used for MIC test for resistant
E. coli isolates after susceptibility test.
Conclusion


Beef handling practices was found to be inadequate to render beef wholesome for human consumption in Jos South LGA. Escherichia coli was found to be prevalent on fresh beef and the E. coli isolates exhibited multidrug resistance. Most antibiotics test in this study may no longer be very effective (> 50% resistance) for treatment of E. coli infection. We recommend that Plateau State government through the Ministry of Agriculture should institute policies that will increase surveillance on zoonotic bacterial agents and antibiotic susceptibilities in food of animal origin in the state. Also, the State should build/organise beef markets and institute proper hygiene in abattoir facilities and for beef handlers.
What is known about this topic
- Resistance in E. coli has been reported to be on increase worldwide
- It is also reported that consumption of food or water contaminated with pathogenic E. coli poses a public health risks to humans
- Escherichia coli of animal origin may act as donors of antimicrobial resistance genes for other pathogenic E. coli and are also identified as indicator for the spread of antimicrobial resistance genes in the environment and to other bacteria
- In Nigeria, bacteriological quality of retailed beef in the market are not ascertained and there is reasonably scarce data relating to the levels of pathogens available in the commercially available fresh beef and non-enforcement of meat regulatory standard in the country
What this study adds
- This study provides baseline information on the beef sellers practices and their awareness on AMR and contribute to the limited information available on the occurrence and antimicrobial susceptibilities of E. coli isolated from retail beef in Jos South LGA of Plateau State
- This study presents the current prevalence of E. coli on beef sold for human consumption
- This study shows E. coli resistance to commonly used antibiotic including the ones used for its treatments
Competing interest


The authors declare no competing interest.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors´ contribution


Iliya Yohanna, Yakubu Gunya Dogonyaro Dashe, Jacob Kwada Paghi Kwaga contributed in the conceptualization of the topic, methodology and writing of the original draft. Jacob Kwada Paghi Kwaga and Godwin Ojonugwa Agada supervised the laboratory investigation. Grace Sabo Kia, Muhammad Shakir Balogun and Charles Michael Akatobi reviewed the manuscript for intellectual content. All the authors read and reviewed the final draft, while Jacob Kwada Paghi Kwaga approved the final manuscript.
Acknowledgments


I am sincerely obliged to Nigeria Field Epidemiology and Laboratory Training Programme and to the Centers for Disease Control and Prevention (CDC) USA, for their support. I am equally indebted to the Department of Veterinary and Pest Control Services of the Federal Ministry of Agriculture and Rural Development for releasing me to embark on this study. I sincerely thank the staff of the Central Diagnostic Laboratory, National Veterinary Research Institute Vom, and my research assistant Mr. Panshak, for their supportive role in the laboratory analysis.
Tables and figures


Table 1: Socio-demographic Characteristics of Beef Sellers in Jos South LGA Plateau State, (n=114)
Table 2: Association between Demographic Characteristics, Practices and Awareness on AMR of beef Sellers in Jos South LGA (n=114)
Table 3: Distribution of E. coli Isolates for Jos South LGA, Plateau State (n=30)
Figure 1: Map of Nigeria Highlighting Plateau State and Jos South LGA as the Study Area
Figure 2: Percentage Resistance of E. coli Isolates Against Antibiotics Disk concentrations in Jos South LGA, Plateau state.
Figure 3: Proportion of Resistance E. coli Isolates against the Number of Antibiotics to which Isolates were Resistant, Jos South LGA Plateau State (n=30)
References


- Lim JY, Yoon J, Hovde CJ. A brief overview of Escherichia coli o157:h7 and its plasmid o157. Journal of Microbiology and Biotechnology[Internet]. 2010 Jan 28[cited 2023 Apr 5]; 20(1):5-14. https://doi.org/10.4014/jmb.0908.08007 Google Scholar
- Zaheer R, Dugat-Bony E, Holman DB, Cousteix E, Xu Y, Munns K, Selinger LJ, Barbieri R, Alexander T, McAllister TA, Selinger LB. Changes in bacterial community composition of Escherichia coli O157:H7 super-shedder cattle occur in the lower intestine. PLoS One [Internet]. 2017 Jan 31[cited 2023 Apr 5];12(1):e0170050. https://doi.org/10.1371/journal.pone.0170050 PubMed | Google Scholar
- Hussein HS. Prevalence and pathogenicity of Shiga toxin-producing Escherichia coli in beef cattle and their products1,2. Journal of Animal Science[Internet]. 2007 Mar 1[cited 2023 Apr 5]; 85(suppl_13):E63-72. https://doi.org/10.2527/jas.2006-421 Google Scholar
- Kaesbohrer A, Schroeter A, Tenhagen BA, Alt K, Guerra B, Appel B. Emerging antimicrobial resistance in commensal Escherichia coli with public health relevance: emerging antimicrobial resistance in commensal e. Coli. Zoonoses and Public Health[Internet]. 2012 Sep[cited 2023 Apr 5]; 59(3):158-65. https://doi.org/10.1111/j.1863-2378.2011.01451.x Google Scholar
- Lupindu AM. Epidemiology of Shiga toxin-producing Escherichia coli O157:H7 in Africa in review. South African J Infect Dis[Internet]. 2018 Jan 2[cited 2023 Apr 5]; 33(1):24-30. https://doi.org/10.1080/23120053.2017.1376558 Google Scholar
- Hammerum AM, Heuer OE. Human health hazards from antimicrobial-resistant Escherichia coli of animal origin. Clin. Infect. Dis[Internet]. 2009 Apr[cited 2023 Apr 5]; 48(7):916-21. https://doi.org/10.1086/597292 Google Scholar
- Yenealem DG, Yallew WW, Abdulmajid S. Food Safety Practice and Associated Factors among Meat Handlers in Gondar Town: A Cross-Sectional Study. J Environ Public Health[Internet]. 2020 Feb 24[cited 2023 Apr 5]; 2020:7421745. https://doi.org/10.1155/2020/7421745 PubMed | Google Scholar
- Kirk MD, Pires SM, Black RE, Caipo M, Crump JA, Devleesschauwer B, Döpfer D, Fazil A, Fischer-Walker CL, Hald T, Hall AJ, Keddy KH, Lake RJ, Lanata CF, Torgerson PR, Havelaar AH, Angulo FJ. World Health Organization Estimates of the Global and Regional Disease Burden of 22 Foodborne Bacterial, Protozoal, and Viral Diseases, 2010: A Data Synthesis. PLoS Med[Internet]. 2015 Dec 3[cited 2023 Apr 5];12(12):e1001921. https://doi.org/10.1371/journal.pmed.1001921 PubMed | Google Scholar
- Kibret M, Abera B. Antimicrobial susceptibility patterns of E. coli from clinical sources in northeast Ethiopia. Afr Health Sci[Internet]. 2011 Aug[cited 2023 Apr 5];11 (3 Spec Iss):S40-5. https://doi.org/10.4314/ahs.v11i3.70069 PubMed | Google Scholar
- Dahiru M, Uraih N, Enabulele S, Shamsudeen U. Prevalence of Eschericia coli 0157:H7 in fresh and roasted beef in Kano City, Nigeria. Bayero J Pure App Sci[Internet]. 2010 Aug 4[cited 2023 Apr 5]; 1(1):43-8. https://doi.org/10.4314/bajopas.v1i1.57513 Google Scholar
- Tafida SY, Kwaga JKP, Bello M, Kabir J, Umoh VJ, Yakubu SE, Nok AJ. Occurrence of Escherichia coli O157 in Retailed-Beef and Related Meat Products in Zaria, Nigeria. FNS[Internet]. 2014 March[cited 2023 Apr 5] ; 05(06):481-7. http://dx.doi.org/10.4236/fns.2014.56057 Google Scholar
- WHO. Antimicrobial resistance[Internet]. WHO; 2021 Nov 17[cited 2023 Apr 5].
- Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterology and Hepatology from Bed to Bench[Internet]. 2013 Jan 14[cited 2023 Apr 5]; 6(1):14-7. https://doi.org/10.22037/ghfbb.v6i1.332 Google Scholar
- Hudzicki J. Kirby-Bauer disk diffusion susceptibility test protocol. American Society for Microbiology[Internet]. 2009 Dec 8[cited 2023 Apr 5]; 66:208. 23 p. Google Scholar
- Andrews J. Detection of extended-spectrum beta-lactamases (ESBLs) in E. coli and Klebsiella species [Internet].British society for antimicrobial chemotherapy; 2012[cited 2023 Apr 5]. Google Scholar
- Andrews JM. Determination of minimum inhibitory concentrations. Journal of Antimicrobial Chemotherapy[Internet]. 2001 Jul 1[cited 2023 Apr 5]; 48(suppl_1):5-16. https://doi.org/10.1093/jac/48.suppl_1.5 Google Scholar
- Isara AR, Isah EC. Knowledge and practice of food hygiene and safety among food handlers in fast food restaurants in Benin City, Edo State. Niger Postgrad Med J. 2009 Sep; 16(3):207-12. Google Scholar
- Rabbi SE, Dey NC. Exploring the gap between hand washing knowledge and practices in Bangladesh: a cross-sectional comparative study. BMC Public Health[Internet]. 2013 Jan 30[cited 2023 Apr 5]; 13(1):89. https://doi.org/10.1186/1471-2458-13-89 PubMed | Google Scholar
- Adesokan HK, Raji AO. Safe meat-handling knowledge, attitudes and practices of private and government meat processing plants' workers: implications for future policy. J Prev Med Hyg[Internet]. 2014 Mar 1[cited 2023 Apr 5]; 55(1):10-6. https://doi.org/10.15167/2421-4248/jpmh2014.55.1.419 PubMed | Google Scholar
- Baş M, şafak Ersun A, Kıvanç G. The evaluation of food hygiene knowledge, attitudes, and practices of food handlers´ in food businesses in Turkey. Food Control[Internet]. 2006 Apr;[cited 2023 Apr 5]; 17(4):317-22. https://doi.org/10.1016/j.foodcont.2004.11.006. Google Scholar
- Altekruse SF, Street DA, Fein SB, Levy AS. Consumer Knowledge of Foodborne Microbial Hazards and Food-Handling Practices. J Food Prot[Internet]. 1996 Mar 1[cited 2023 Apr 5]; 59(3):287-94. https://doi.org/10.4315/0362-028x-59.3.287 Google Scholar
- Ansari-Lari M, Soodbakhsh S, Lakzadeh L. Knowledge, attitudes and practices of workers on food hygienic practices in meat processing plants in Fars, Iran. Food Control[Internet]. 2010 Mar[cited 2023 Apr 5]; 21(3):260-3. https://doi.org/10.1016/j.foodcont.2009.06.003 Google Scholar
- Oloso NO, Fagbo S, Garbati M, Olonitola SO, Awosanya EJ, Aworh MK, Adamu H, Odetokun IA, Fasina FO. Antimicrobial Resistance in Food Animals and the Environment in Nigeria: A Review. Int J Environ Res Public Health[Internet]. 2018 Jun 17[cited 2023 Apr 5];15(6):1284. https://doi.org/10.3390/ijerph15061284 PubMed | Google Scholar
- Kamata K, Tokuda Y, Gu Y, Ohmagari N, Yanagihara K. Public knowledge and perception about antimicrobials and antimicrobial resistance in Japan: A national questionnaire survey in 2017. PLoS One[Internet]. 2018 Nov 5[cited 2023 Apr 5]; 13(11):e0207017. https://doi.org/10.1371/journal.pone.0207017 PubMed | Google Scholar
- WHO. Global action plan on antimicrobial resistance[Internet]. WHO; 2016 Jan 1[cited 2023 Apr 5].
- WHO. 2018-Pakistan: Typhoid fever - Islamic Republic of Pakistan[Internet]. WHO; 2018 Dec 27[cited 2023 Apr 5].
- Azhar AB, Khalid A, Shah S. The Implications of Extensive Drug-resistant Typhoid Fever: A Case Report. Cureus[Internet]. 2019 Jun 29[cited 2023 Apr 5]; 11(6):e5032. https://doi.org/10.7759/cureus.5032 PubMed | Google Scholar
- Cho H, Salmon CT. Unintended Effects of Health Communication Campaigns. J Commun[Internet]. 2007 Jun 1[cited 2023 Apr 5]; 57(2):293-317. https://doi.org/10.1111/j.1460-2466.2007.00344.x Google Scholar
- Koffi-Nevr R, Koussemon M, Coulibaly SO. Bacteriological quality of beef offered for retail sale in cote d´ivoire. American J of Food Technology[Internet]. 2011 Aug 15[cited 2023 Apr 5]; 6(9):835-42. https://doi.org/10.3923/ajft.2011.835.842 PubMed | Google Scholar
- WHO. WHO First Ever Global Estimates of Foodborne Diseases Find Children Under 5 Account for Almost One Third of Deaths[Internet]. WHO; 2015 Dec 3[cited 2023 Apr 5].
- Søraas A, Sundsfjord A, Sandven I, Brunborg C, Jenum PA. Risk factors for community-acquired urinary tract infections caused by ESBL-producing enterobacteriaceae--a case-control study in a low prevalence country. PLoS One[Internet]. 2013 Jul 23[cited 2023 Apr 5]; 8(7):e69581. https://doi.org/10.1371/journal.pone.0069581 PubMed | Google Scholar
- Odonkor ST, Addo KK. Microbiological quality of water sources from the largest district in Greater-Accra region, Ghana: a call for innovational schemes towards rural water resources management[Internet]. International Journal of Science, Environment and Technology. 2013 Aug 2[cited 2023 Apr 5]; 2(4): 536 - 555. Google Scholar
- WHO. Antimicrobial Resistance: Global Report On Surveillance[Internet]. Geneva: WHO; 2014[cited 2023 Apr 5].
- Navarro-Gonzalez N, Porrero MC, Mentaberre G, Serrano E, Mateos A, Domínguez L, Lavín S. Antimicrobial resistance in indicator Escherichia coli isolates from free-ranging livestock and sympatric wild ungulates in a natural environment (Northeastern Spain). Appl Environ Microbiol[Internet]. 2013 Oct[cited 2023 Apr 5];79(19):6184-6. https://doi.org/10.1128/AEM.01745-13 PubMed | Google Scholar
- Natarajan M, Kumar D, Mandal J, Biswal N, Stephen S. A study of virulence and antimicrobial resistance pattern in diarrhoeagenic Escherichia coli isolated from diarrhoeal stool specimens from children and adults in a tertiary hospital, Puducherry, India. J Health Popul Nutr[Internet]. 2018 Jul 13[cited 2023 Apr 5];37(1):17. https://doi.org/10.1186/s41043-018-0147-z PubMed | Google Scholar
- Miranda EJ, Oliveira GS, Roque FL, Santos SR, Olmos RD, Lotufo PA. Susceptibility to antibiotics in urinary tract infections in a secondary care setting from 2005-2006 and 2010-2011, in São Paulo, Brazil: data from 11,943 urine cultures. Rev Inst Med Trop Sao Paulo[Internet]. 2014 Jul-Aug[cited 2023 Apr 5];56(4):313-24. https://doi.org/10.1590/s0036-46652014000400009 PubMed | Google Scholar
- Najjuka CF, Kateete DP, Kajumbula HM, Joloba ML, Essack SY. Antimicrobial susceptibility profiles of Escherichia coli and Klebsiella pneumoniae isolated from outpatients in urban and rural districts of Uganda. BMC Res Notes[Internet]. 2016 Apr 25[cited 2023 Apr 5]; 9(1):235. https://doi.org/10.1186/s13104-016-2049-8 PubMed | Google Scholar
- Kidsley AK, Abraham S, Bell JM, O'Dea M, Laird TJ, Jordan D, Mitchell P, McDevitt CA, Trott DJ. Antimicrobial Susceptibility of Escherichia coli and Salmonella spp. Isolates From Healthy Pigs in Australia: Results of a Pilot National Survey. Front Microbiol[Internet]. 2018 Jul 9[cited 2023 Apr 5];9:1207. https://doi.org/10.3389/fmicb.2018.01207 PubMed | Google Scholar
- Clinical and Laboratory Standards Institute. Performance standards for antimicrobial disk susceptibility test; approved standard. 12th ed. Patel JB, Cockerill 3rd FR, Bradford PA, Eliopoulos GM, Hindler JA, Jenkins SG, Lewis 2nd JS, Limbargo B, Miller LA, Nicolau DP, Powell M, Swenson JM, Traczewski MM, Turnidge JD, Weinstein MP, Zimmer BL, editors. Wayne, PA: Committee for Clinical Laboratory Standards; 2015 Jan. (Documents / Clinical and Laboratory Standards Institute). CLSI document M02-A12. Google Scholar