Resource mapping and Malaria surveillance capacity of health facilities in Federal Capital Territory, Abuja, Nigeria-June 2020
Henry Uguru Ekechi1,2,3,&, IkeOluwapo Ajayi4, Aderemi Oludiran Kehinde5, Chukwuma David Umeokonkw6, Chioma Dan-Nwafor2, Celestine Ameh7, Muhammad Shakir Balogun7
1Nigeria Field Epidemiology and Laboratory Training Programme, 2Nigeria Centre for Disease Control (NCDC), 3Department of Public Health, Health and Human Services Secretariat, FCTA, Abuja, 4Department of Epidemiology and Medical Statistics, University of Ibadan, 5Department of Medical Microbiology and Parasitology, University of Ibadan, 6Alex Ekwueme Federal University Teaching Hospital, Abakaliki, Nigeria, 7African Field Epidemiology Network
&Corresponding author
Henry Uguru Ekechi, Nigeria Field Epidemiology and Laboratory Training Programme, Nigeria Centre for Disease Control (NCDC), Federal Ministry of Health
ekechi81@gmail.com
Introduction:
Malaria is responsible for about 500 million cases and one million deaths annually. A disease of high burden in sub-Saharan Africa, with challenges of resource deficit leading to delayed elimination, we determine resources available in health facilities for malaria surveillance, identified gaps, and made recommendations towards malaria elimination in Nigeria.
Methods:
Cross-sectional survey was conducted to determine the availability of malaria surveillance tools and officers, their knowledge, and the laboratory capacity of facilities. Knowledge was graded against a total score of 100 percent and ?70 percent score was considered good knowledge. Descriptive, bivariate, and multivariate analysis was done, and the 95% confidence interval of the adjusted odds ratio were reported.
Results:
A total of 221 facilities were studied. Mean age of respondents was 37.6 years ± 8.4, and 66.0% were males. All facilities studied had malaria focal persons and 85.5% were Community Health Officers (CHOs). Eighty-eight percent had good knowledge of malaria surveillance. Being a Nurse (p=0.004), Record Officers (p=0.03), and a duration of 1-5years as Malaria surveillance in-charge (p<0.001) were associated with, and predicting factors for good knowledge. While 95.0% report on indicators timely, 90.0% routinely analyzed their data. Surveillance tools available in facilities were outpatient and inpatient registers, outpatient and inpatient patient's card, and reporting forms. Only 16.3% of facilities had computers, while 59.0% of facilities had a functional laboratory with about half of the treated malaria cases laboratory confirmed.
Conclusion:
Resources and capacity for malaria diagnosis within the health facilities were sub-optimal. Conscious and deliberate efforts through training, equipping, and enhanced supportive supervision would be required to improve the system towards malaria elimination.
Introduction

Malaria is one of the important vector-borne diseases with high fatality rates in tropical countries [1]. It is caused by the bites of mosquitos of the Anopheles spp. causing plasmodium infection in humans. Globally, malaria is responsible for about 500 million cases and one million deaths each year [2]. In 2017, there were an estimated 219 million cases of malaria with 435,000 in 87 countries. The WHO African Region carries a disproportionately high share of the global malaria burden. In 2017, the region was home to 92% of malaria cases and 93% of malaria deaths [3]. It has consistently remained a disease of high burden in Africa and most developing countries. As a disease of high burden, it impacts negatively on the economy of the already struggling developing countries most of which are in Africa [4]. Malaria is highly endemic in Nigeria where young children and pregnant mothers are the most affected according to reports [5]. African Heads of states, met in Abuja, Nigeria on April 25, 2000, to express commitment to the Roll Back Malaria (RBM) initiative seeing the economic burden the disease has placed on the continent [2,6]. Malaria Surveillance was identified as key to reducing the burden of malaria and its control in the meeting.
Effective surveillance is required at all points from malaria control to malaria elimination. The human and non-human resources are among the component of an effective surveillance system which consists of the tools, procedures, people, and structures [
7]. Challenges of inadequate structures, lack of surveillance tools, noncompliance to existing procedures by program officers, general resource deficit, and suboptimal capacity of program officers to malaria surveillance have been highlighted in some studies [
8]. Hence, efforts to eradicate malaria has been elusive with planning and intervention activities very challenging [
9]. Globally, the ineffective malaria surveillance with poor resources have translated to poor program outputs. A report of seventeen countries in Africa involved in a malaria surveillance system evaluation showed that 14(82%) countries use paper-based collection of incidence data at the health facilities with 80% of cases treated without laboratory diagnostic information [
10]. In the countries where electronic data collection was practices 3(18%), the extent at which this electronic data collection occurred varied among countries [
10,
11]. Across Nigeria, studies showed that malaria surveillance resources and the quality of laboratory testing need to be improved and ensure the quality of laboratory confirmation especially in high transmission areas [
12].
As observed during supportive supervisions, resources in malaria programs are identified as a ‘weak point&rsqup; towards malaria elimination in Nigeria. However, the extent of this is yet to be determined in parts of the country including Federal Capital Territory (FCT). This has impacted negatively the interventions towards eliminating malaria in Nigeria. Through this study, we hoped to identify the proportion of resource challenges and make recommendation based on findings to improve the system.
Methods


Participants
All 221 public health facility in FCT which has been in operation for at least one year were included in the study while those with interrupted services at the time of the study, were excluded from the study. The private health facilities were also excluded due to their uneven spread in the territory.
Study setting and design
We conducted a cross-sectional study and mapping of resources for malaria surveillance in 221 public health facilities in FCT between June - August, 2020.
Our study setting is the FCT in Nigeria with a landmass area of 7,315 sq.km, of which the definite city occupies 275.3 sq.km [
12]. FCT has a tropical wet and dry climate [
13]. The city experiences three weather conditions annually; warm, humid rainy season, and a blistering dry season [
14]. The rainy season begins from April and ends in October.
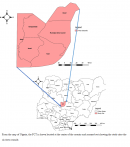
FCT located at the center
Figure 1 of the country, Nigeria, and has a projected population of 4,464,785 according to the National Population Commission census with males accounting for (52%) at 9.3% annual growth rate [
14]. It is served by approximately 257 public health facilities; 3 Tertiary, 14 Secondary, and 240 Primary Health Care (PHC) facilities; and about 250 private health facilities. This study was conducted in the Six Area Councils (Municipal, Bwari, Gwagwalada, Kuje, Kwali and Abaji) of the Federal Capital, targeting the public health facilities where one MFP is expected to routinely collect, collate, analyze and report to the next level in the reporting channel.
A pretested structured interviewer-administered questionnaire was used to determine availability of malaria surveillance tools and officers, their knowledge and laboratory capacity in the 221 health facilities.
Sample size determination
We achieved a representative sample size for the study after having estimated the required minimum sample size at a 95% confidence interval and prevalence of 50 %, since no information exist in any previous study on this research topic [
15].
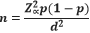
n = minimum sample size
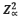
= Standard normal deviate. (At 5% type 1 error where (p<0.05), standard normal deviate is 1.96
p = 0.5. For this study, 0.5 was used since no information exist in any previous study on this research topic [
15].
d= Absolute error or precision indicating how close the proportion of interest is to the desired estimate, we used 0.05 in this study;
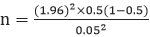
= 384
Finite population correction for proportions
With target population is 257(≈ approximate number of public health facilities in the FCT) we sampled without replacement, applying finite population correction.
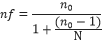
n = Minimum sample size calculated (384)
N = Total population of public health facilities in FCT
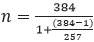
= 155
A design effect factor of 1.5 was considered based on anticipated clustering of
responses due to the sampling technique used.
(1.5 x 155) = 232.5
Allowing for non-response rate of 5% (232.5/0.95) = 221 ref [
16].
The minimum sample size of this study was therefore estimated at 221.
Sampling technique
A stratified cluster sampling technique was used. The clusters were the primary health facility units of the various Area Councils.
The list of all the PHCs were grouped according to Area Councils as sampling frames. There were 70 PHCs (Municipal), 36 PHCs(Bwari), 35PHCs(Gwagwalada), 34PHCs(Abaji), 33PHCs(Kwali), and 32PHCs(Kuje) as obtained from the routine report of the diseases of Public health importance in FCT. From table of random numbers, we selected randomly 52 PHCs from Municipal, 31(Bwari), 31(Gwagwalada), 30(Abaji), 30(Kwali), and 30(Kuje) (based on the proportion of reporting facilities by each Area council in the routine monthly reporting platform) making a total of 204 PHCs. All 14 secondary and 3 tertiary health facilities were purposively selected for the survey. We obtained a total of 221 health facilities (204PHCs, 14 secondary, and 3 tertiary health facilities). In the PHCs the respondents were persons responsible for malaria surveillance. One respondent from each health facility
Data collection
Research assistants were trained to reduce inter-observer variation in data collection. They were regularly supervised to ensure quality data collection. In each facility, pretested structured questionnaires were administered to the in-charge malaria surveillance. Assessment checklist was used to determine which malaria surveillance tool was available in the facilities; this was used as a check on the responses provided by the respondents. All the interviews were conducted privately to ensure confidentiality after obtaining their written informed consents.
This questionnaire was developed based on the specific objectives of this research work, considering the resources for malaria surveillance to be mapped as identified during the pre-mapping stage of resource mapping.
The resources mapped in this study are the specific employees in-charge of malaria surveillance in the facilities, equipment, documents and materials directly involved in the conduct malaria surveillance activities in the facilities. Other resources outside the scope of this study were excluded based on compliance with the study protocol.
The questionnaire consisted of the sociodemographic data of respondents, respondents´ knowledge and capacity for malaria surveillance, malaria surveillance tools and laboratory capacity for diagnosis.
Data analysis
Descriptive and analytical statistics were used to summarize the data obtained. Knowledge on malaria surveillance (summary of responses to knowledge indicator variables (suspected and confirmed case definitions, presentation of malaria cases (severe, non-severe), components of complicated malaria, and routine reporting of malaria indicators) was graded against a total score of 100 percent and ≥70 percent score was considered good knowledge (scores ≥70 percent is academically considered an upper limit grade score), otherwise poor knowledge. Findings were summarized and presented in tables, charts and maps. Continuous variables were summarized using mean, range, and standard deviation. Frequencies and proportions were used to summarize categorical variables. In bivariate analysis, fishers exact test (It´s appropriate to use Fisher´s exact test, in particular when dealing with small counts) was used to test associations between categorical outcome variables and explanatory variables. Multivariate analysis was conducted using multiple logic regression to determine predictors of knowledge of malaria surveillance. The level of significance was set at 5%.
Ethical Considerations
Ethical approval was obtained from the FCT Health Research Ethics Committee FHREC/2020/01/12/18-02-20 and written informed consent was obtained from each respondent before participating in the study.
Results


Sample characteristics
A total of 221 health facilities participated in this study; with one respondent from each facility, a mean age of 37.6 ± 8.4 years and age range of 20-59 years. The facilities sampled were evenly spread (see Figure 2). Two Hundred and Four (92.0%) of facilities were primary facilities, secondary facilities 14(6%), and tertiary 3(2%). Among the respondents, 145 (66.0%) were male, 119 (53.9%) were aged greater than 35 years of age, 82 (37.1%) were of the Gbagyi ethnicity, 211 (95.5%) attended tertiary institutions, 181(81.9%) were married among the respondents. Other demographic characteristics are shown in
Table 1.
Availability of Malaria Surveillance Officers in the Health Facilities
Among the 221 facilities surveyed, 160(72.4%) had their facility in-charge double as in-charge of malaria surveillance activities, while in other facilities, they were, record officers 22(10%), malaria focal persons 18(8.1%), and surveillance focal persons 10(4.5%)
Table 2. Some (131, 59.3%) of the respondents have been in-charge of malaria activities for 1-5 years, 66 (29.9%) of them for 6-10 years, 20(9%) others for more than 10years while only 4(1.8%) have been in-charge for less than one year. Majority (189, 85.5%) were Community Health Officers, 15(6.8%) Nurses, 9(4%) Record Officers, 3(1.4%) Medical Officers, and others (2.3%) comprising Scientific Officers, Laboratory Scientist and Cashier
Table 2.
Malaria Surveillance Officers´ Knowledge of Malaria Surveillance
One hundred and ninety-five respondents (88.2%) understood suspected malaria cases while 132 (59.7%) understood confirmed malaria cases. Most of them (99.0%) understood how malaria cases present. Some (164, 74.2%) of respondents understood the signs and symptoms. One hundred and forty-eight (67.0%) defined correctly what routine reporting in Malaria surveillance is
Table 3).
Malaria Surveillance Officers´ Capacity on Malaria Surveillance
Two hundred and nine (95%) sent reports timely. One Hundred and eighty-Five (83.7%) keep the ‘monthly malaria report’. None of the surveyed health facilities had a ‘list of foci’ and ‘entomological database’.
Among 221 respondents, 184 (83.6%) analyzed malaria indicators monthly, 11(5.0%) quarterly, 3(1.4%) annually, 1(0.4%), while 21(9.6%) do not
Figure 2. Sixty percent of those who carry out analysis, use the analysis in malaria intervention activities, 80 (40.0%) did not use the analyzed data. Some of the activities includes determining effective malaria drug within the catchment area which is served by the facility 18(15.1%), determining burden and trends of cases overtime 25(21.0%), generating reports for policymaking 29(24.5%), and other malaria intervention activities 34(28.6%), health education & awareness creation on malaria prevention 40(33.6%) and guide the distribution of LLITN and other malaria commodities to end-users 21(17.6%)
Figure 3.
Available Malaria Surveillance Tools in the Health Facilities
Availability of malaria surveillance tools showed that, only 36 facilities (16.3%) have computers, 15(6.8%) case investigation forms, 106 (48.0%) reporting forms, 55 (24.9%) tally sheets, 158(71.5%) registers, 114(51.6%) patients´ cards, 9(4.1%) dashboards, and 13(5.9%) training materials.
Most of the computers 22(61.1%) available during the survey were found in the facilities from Municipal Area Council, others were from Abaji 8(22.2%) and Bwari 6(16.7%). All 3(100%) tertiary facilities studied had computers while the rest were available in 10(71.4%) secondary facilities and 23(11.3%) primary health facilities. Only 1(0.5%) facility had all the surveillance tools while 24(10.9%) facilities had at least one of the surveillance tool.
In aggregate, Abaji, Bwari and Gwagwalada Area Councils had more of the tools (2 to 3 tools per facility), with Municipal, Kuje and Kwali Area Council having less (1 to 2 tools per facility)
Figure 4.
Laboratory Capacity for Malaria Diagnosis and Proportion of Tested/Untested Malaria Cases
One hundred and thirty-two (59.9%) facilities had a functional laboratory (in-use during the survey), out of which 113 (85.6.%) had the capacity to conduct microscopy tests. Some of the facilities with functional laboratories 108(81.8%) carry out other confirmatory tests such as the RDT, serological and, quantitative buffy coat. Eleven (5.0%) of the all the facilities studied perform only microscopy test confirmatory procedure, while 95 (43%) conduct either microscopy, RDT or other confirmatory tests. During this study, 19,544 cases of malaria cases were treated in the health facilities surveyed. About half 10,143 (51.9%) were confirmed using either microscopy test 1,014 (7.0%), RDT 9,118(89.95%), serological test, or quantitative buffy coat technique.
Association between Respondents´ Malaria Surveillance Knowledge and other Characteristics
Significant associations were found between the respondents´ level of education, designation, duration of years as malaria in charge, and their malaria surveillance knowledge. The odds of MFP having good malaria surveillance knowledge was 6 times less if the MFPs´ level of education is ‘secondary’ (Secondary vs Tertiary; OR: 0.94; 95% CI: 0.19,0.98) and 77 times less when the MFPs´ duration as malaria surveillance in-charge is <1 year (<1year vs >10years; OR: 0.23; 95% CI: 0.12,0.67).
The odds of MFPs having good malaria surveillance knowledge was also about 5 times more when the MFPs´ designation is ‘nursing’ (Nurse vs CHO; OR: 4.83; 95% CI: 1.63,14.3), 4 times more when their designation is ‘Record Officer’ (Record Officer vs CHO; OR: 4.41; 95% CI: 1.12,17.4) and 3 times more when MFPs´ duration as malaria surveillance in-charge is 1-5 years (1-5years vs >10years; OR: 3.42; 95% CI: 1.76,7.24)
Table 4.
Predictors of Malaria Focal Persons´ Malaria Surveillance Knowledge
After adjusting for confounders, having a Nurse, Record Officer as in charge of Malaria surveillance and being in-charge of Malaria surveillance for 1-5 years remained significantly associated with good knowledge of malaria surveillance
Table 4.
The odds of Malaria surveillance in-charge who were Nurses having good knowledge of malaria surveillance were 4 times more than other cadres (aOR: 4.71, 95% CI: 1.58, 13.9), the odds of in-charges who were Record Officers having good knowledge of malaria surveillance were 5 times more than other cadres (aOR: 5.38,95% CI: 1.27, 22.7) and the odds of those in-charges who had been in the position for 1-5 years having good knowledge were 3 times more than those who have been in-charge at different durations (aOR: 3.40, 95% CI: 1.65, 7.34)
Table 4.
Association between other characteristics and Non-Availability of Malaria Surveillance Tools in the Facilities
Significant associations were found between the location of facilities, and non-availability of malaria surveillance tools. The odds of not having malaria surveillance tools in the facilities were 61 times less if a facility is located in ‘Kwali Area council’ (Kwali vs Abaji; OR: 0.39; 95% CI: 0.26,0.59), 36 times less if a facility is located in ‘Kuje Area council’ (Kuje vs Abaji; OR: 0.64; 95% CI: 0.44,0.93) and 33 times less if a facility is located in ‘Municipal Area council’(Municipal vs Abaji; OR: 0.67; 95% CI: 0.49,0.93). While the odds having malaria surveillance tool available in a facility was about one time more when the facility is situated in ‘Bwari’ (Bwari vs Abaji; OR: 1.03; 95% CI: 0.72, 1.47).
Table 5.
Predictors of Availability Malaria Surveillance Tools
After adjusting for confounders, having a health facility in Kwali Area Council remained significantly associated with non availability of malaria surveillance tools in the facilities (see Table 5). The odds of facilities situated at Kwali Area Council not having malaria surveillance tool were 66 times less than other Area Councils (aOR: 0.34, 95% CI: 0.44, 2.46).
Discussion


Malaria surveillance is critical to achieving malaria elimination goals. Human and nonhuman resources contribute to the strategic goals of malaria surveillance. According to WHO; “Malaria surveillance system consists of the tools, procedures, people and structures [17]. In this study, we observed the availability of persons responsible for malaria surveillance activities in the health facilities. In most of the surveyed facilities, the facility in-charges doubled as the malaria surveillance in-charge. The cadres of other malaria surveillance officers in this study were Record Officers, DSNOs, and MFPs. Canavati et al., 2016 suggested in their studies that various cadres of officers in the health profession can function as malaria surveillance officers, where such officers are trained [11,18]. Ruebush et al., agreed with them but stated that, having officers dedicated only to the work of malaria surveillance, will yield a preferred result with maximum service delivery [18,19]. Unfortunately, only 8.2% of the malaria officers in our study were MFPs.
More than half (59.3%) of the respondents had been in-charge of malaria surveillance for 1-5 years. This finding is consistent with studies conducted by Olugbade et al., 2012 and Kureya et al., 2016, but contrary to Tyakaray et al., 2020. Tyakaray in her study observed that most of the MFPs had been in the position of malaria surveillance officers for 11-15 years [
20,
21]. Their study did not determine the reason for this disparity.
Most of the malaria surveillance officers were CHOs, which is similar to the finding of Tyakaray et al, 2020; where majority (91.4%) of the malaria surveillance officers in their study were CHOs. The cadre of the malaria surveillance officers in our study varied including Nurses, Record Officers and Medical Doctors [
20,
21]. Having most respondents in this study as CHOs was not a surprise considering the study of Slavea et al, and Abimbola et al. These two studies showed that CHOs/CHEWs are the most frequently found cadre of health professionals in primary health facilities [
22,
23].
Knowledge assessment of the respondents on malaria surveillance showed that they have good knowledge in some aspect (index of suspicion) of malaria, but poor knowledge of routine reporting and the role of laboratories in confirmation of malaria cases. This is similar to the outcome of the study by Sumadhya et al., 2015 in Srilanka but contrary to the study by Aniwada et al., 2016 in Enugu Nigeria where the study participants showed very good knowledge of malaria surveillance [
24,
25].
Respondents who mentioned correctly the presentation of a ‘suspected malaria case’ indicates good knowledge. This finding was not a surprise since all the respondents function within the facilities and would have witnessed cases first hand. This finding is in agreement with the study by Ladi-Akinyemi et al., where a ‘higher percentage of healthcare workers had good knowledge on the mode of transmission of malaria and signs and symptoms of simple malaria’. The findings of our study however, are in disagreement with Ladi-Akinyemi et al., who found that ‘less than half and one-third of the heath-care workers had good knowledge of case definition of simple and complicated malaria respectively ’[
26].
Those who understood clearly, ‘routine reporting’ were below the 80% minimum benchmark of ‘World Malaria Report 2018 ’and ‘Malaria Consortium Project Brief’ on reducing the malaria burden in Nigeria, 2018 [
3]. However, in practice, the respondents in this study exceeded the minimum required benchmark of 80 percent expected in routine reporting. According to Avong et al., 2018, training and continuous training would greatly improve the knowledge and frequency of reporting as they observed 100 percent improvement among participants in the frequency of reporting after a training exercise compared to the status of reporting before the training [
27].
Our study equally showed that awareness of how often reports are to be sent to the next level was good (90.4 percent), however, they were 9.6 percent of respondents who do not know they should report to the next level at all. In similarity to the study by Ofili et al., 2003; only 11.9 percent of surveyed healthcare workers had a good knowledge of disease notification [
28].
The availability of malaria surveillance tools showed that, 36 facilities (16.3%) had computers for malaria surveillance activities and other tools in varied degrees. Robust and responsive information systems are critical for successful malaria control and elimination [
29]. According to Ohrt et al., computers are undoubtedly the most important tool in malaria surveillance with the potentials of integrating all other paper-based tools thereby making management and reporting almost seamless. Unfortunately, only 16.3 percent of the health facilities in our study had computers for malaria surveillance activities. This is consistent with findings of Alwan et al, 2015, which showed that computer availability was generally low in the facilities surveyed in their study and was lower for public primary health care centers [
30,
31]. This finding is critical to malaria surveillance, control, and elimination. However, in our study the availability was low.
Furthermore, other paper-base tools like case investigation forms, tally sheets and reporting forms were not available in some of the facilities at the time of this study. The reason for the absence of these documents might be as a result of stock out of commodities in the face of COVID-19 pandemic response, as more attention was channeled towards the pandemic at the detriment of other diseases of public health importance. Nghochuzie et al, 2020; agreed completely with this possibility in their study, ‘Pausing the Fight Against Malaria to Combat the COVID-19 Pandemic in Africa: Is the Future of Malaria Bleak?’. Their study showed that they occurred a geometric rise in the malaria mortality and morbidity during the COVID-19 pandemic across some African countries partly due to shortage in the commodities and consumable used in malaria elimination programs across the continent [
32]. Rogerson et al, 2020 also corroborated the findings of Nghochuzie in their study, �Identifying and combating the impact of COVID-19 on malaria´. They stated that scarcity of commodities and consumable for malaria response suffered greatly, more so, as companies shifted their attention from production of malaria related consumables to COVID-19, especially in the low and middle income countries(LMIC) [
33].
As observed in our study, ‘list of loci’ and entomological database were not found in any of the facilities. The reason for the absence of these tools in the facilities was not determined in the study, however, it may not be unconnected with the findings of Rumisha et al, 2014. In their study, they mentioned that the collection of entomological data of mosquitos across African countries of Tanzania, Kenya, Mozambique, Senegal, Ghana and Burkina Faso was the only of such data available in Africa [
34]. Malaria transmission is measured using entomological inoculation rate (EIR) [
34], without which, understanding the heterogeneity of malaria transmission will be difficult; the understanding of which required in the process of malaria eradication and elimination process. Chanda et al disagreed with the scarcity of the entomological data in the continent through their study, ‘Operational scale entomological intervention for malaria control: strategies, achievement and challenges in Zambia’ [
35]. He clearly stated that capacity and data to undertake entomological assessment is available in Zambia, but added however that it is an aspect of malaria eradication strategy which will require strengthening if malaria control will be successful in the Continent. In laboratory capacity across the facilities, 132 (59.9%) had functional laboratory and 113 (51.1%) conduct microscopy tests. This finding is consistent with Kyabayinze et al., finding in Uganda where health facilities had inadequate resource capacity for effective health care delivery including parasite-based malaria diagnosis. In their study, 24% of Health facilities had malaria diagnostics, 29% had functional microscopy and 20% had only RDTs in use(36). Our study differed from the findings of Abreha et al, 2014; in Ethiopia where most of the health facilities had basic infrastructure and equipment to perform malaria laboratory test [
37].
One of the findings of our study is that, confirming malaria using the RDT procedure is quite a popular practice among health facilities in the FCT. That is consistent with the findings of Wilson et al, where, the most commonly used method for identifying causes of malaria remains the microscopic examination, however, the authors stated that there is growing use of malaria rapid diagnostic test in many parts of the world [
38].
In this study, about half (51.9%) of the treated malaria cases were confirmed using a confirmatory test, which is similar to the findings of Fraol et al, 2014; in Ethiopia, where 67.4% of the treated malaria cases were confirmed. The author alluded that lack of training and low work experience of laboratory professionals were factors associated with malaria microscopy diagnostic performance [
39]. In contrary, Bamiselu et al., 2016; in Ogun State Nigeria, showed high level of compliance to treatment of most (98.1%) malaria cases after the confirmatory test in their study. The authors suggested that awareness of national treatment guidelines was responsible for this level of compliance among health workers in both public (98.1 %) and private (94.8 %) settings [
40].
The limitations we encountered in this study include the study design, social desirability bias and common method bias. It was difficult to determine causality between independent and dependent variables due to the inability to fulfill time sequence criteria. Predictors found were only considered suggestive. The study design was limiting in the sense that the study was carried out at one-time-point and gives no indication of sequence of events. Where all the outcome factors are difficult to determine when they occurred; whether before, after or during the process that was studied. This being so, the predictors found can only be suggestive. The social desirability bias was mitigated by training the research assistant to adopt indirect questioning of the respondents where necessary, while the common method bias was mitigated by training the research assistants on asking the questions using procedures which made the respondents to provide answer in easy and non-stylish manner. The respondents were encouraged to provide answers based on the information within their purview.
The average distribution of the malaria surveillance tools across Area councils and health facility types which showed more tools available in the secondary and tertiary health facilities on buttressed the neglect of the primary level of healthcare in the country. Abdulrahaman et al, 2012 and Alenoghena et al, 2014 unanimously presented in the study the obvious neglect of the primary healthcare system in Nigeria. They both called on the authorities to reform the healthcare system where the primary healthcare system will be repositioned as the first and closest healthcare system to the communities [
41,
42].
Conclusion


Human and nonhuman resources were available for malaria surveillance activities within the health facilities in the FCT, however, they were suboptimal. Most of the MFPs were not dedicated solely to the duty of malaria surveillance. The knowledge and practice of malaria surveillance among some MFPs was poor but being a Nurse, Record Officer, and being in position as in-charge malaria surveillance up to five years may improve the knowledge and practice of officers in malaria surveillance activities. Near absence of basic tools like computers for malaria surveillance, empirical treatment of many malaria cases which may lead to development of resistant strains and suboptimal laboratory capacity, underscores the need to review and reposition the program of malaria elimination in the FCT to achieve the set program goal of malaria eradication.
What is known about this topic
- There is Malaria Surveillance System (MSS) under the Malaria Elimination Program (MEP) in the Federal Capital Territory, Nigeria
- There have been concerted effort to eliminate malaria in Nigeria and other developing countries of the World.
What this study adds
- The study reveals the spread and designation of the personnel in charge of MSS in the facilities across the Territory
- The study further shows the capacity of the personnel, tools available for their operation and the predictor of knowledge of these officers involved in MSS
- The study also identified gaps in the MSS and made valuable recommendation for improvements.
Competing interests


The authors declare no competing interests.
Authors' contributions


Henry Uguru Ekechi –Conception, Data collection, analysis, drafting and editing. Ajayi IkeOluwa – Conception, Manuscript review and Editing. Aderemi Kehinde –Manuscript review and Editing. Umeokonkwo David Chukwuma –Manuscript review and analysis. Dan-Nwafor Chioma – Manuscript review. Ameh Celestine – Manuscript review and analysis. Balogun Muhammad Shakir – Administration and review.
Acknowledgments


We acknowledge the contributions of the FCT Disease Surveillance and Notification Officers(DSNOs), who, even in the heat of COVID-19 pandemic were in the field collecting data for this study.
Tables and figures


Table 1: Sociodemographic Characteristics of the Respondents (N=221)
Table 2: Frequency Distribution of Malaria Surveillance Officers in the Health Facilities (N=221)
Table 3: Malaria Surveillance Officers´ Knowledge of Malaria Surveillance (N=221)
Table 4: Respondents Characteristics Significantly Associated with the Knowledge of Malaria Surveillance
Table 5: Characteristics Significantly Associated with the Availability of Malaria Surveillance Tool
Figure 1: Nigeria Map showing FCT.
Figure 2: FCT Map showing distribution/location of the health facilities that participated in the study.
Figure 3: Percentage distribution of activities carried out Using the findings of the analyzed malaria indicators.
Figure 4: Distribution of the Average number of Malaria Surveillance Tools by Facilities in the Six Area Councils.
References


-
Kaur H, Sehgal R, Kumar A, Sehgal A, Bharti PK, Bansal D, Mohapatra PK, Mahanta J, Sultan AA. Exploration of genetic diversity of Plasmodium vivax circumsporozoite protein ( Pvcsp ) and Plasmodium vivax sexual stage antigen ( Pvs25 ) among North Indian isolates. Malar J[Internet]. 2019 Sep 6[cited 2022 Dec 26] ;18(1):308. https://doi.org/10.1186/s12936-019-2939-z . PubMed | Google Scholar
- Chukwuocha UM. Malaria control in Nigeria. Primary Health Care[Internet]. 2012 Jun 23[cited 2022 Dec 26]; 02(03). https://doi.org/10.4172/2167-1079.1000118 Google Scholar
- World Health Organization (WHO). World malaria report 2018[Internet]. Geneva: World Health Organization; 2018[cited 2022 Dec 26]. 166 p. PubMed | Google Scholar
- Mills A, Lubell Y, Hanson K. Malaria eradication: the economic, financial and institutional challenge. Malar J[Internet]. 2008 Dec 11[cited 2022 Dec 26]; 7(S1):S11. https://doi.org/10.1186/1475-2875-7-S1-S11 PubMed | Google Scholar
- Selvaraj P, Suresh J, Wenger EA, Bever CA, Gerardin J. Reducing malaria burden and accelerating elimination with long –lasting systemic insecticides: a modelling study of three potential use cases. Malar J[Internet]. 2019 Sep 5[cited 2022 Dec 26];18(1): 307. https://doi.org/10.1186/s12936-019-2942-4 . PubMed | Google Scholar
- Okeke EU. Nigerian malaria: the problems and the fight. Malar J[Internet]. 2012 Oct 15[cited 2022 Dec 26];11(S1):P122. https://doi.org/10.1186/1475-2875-11-S1-P122 PubMed | Google Scholar
- USAID. President�s Malaria Initiative:Nigeria-Malaria Operational Plan FY 2018[Internet]. USAID; 2017 Nov[cited 2023 Jan 30].
- Pasquale H, Jarvese M, Julla A, Doggale C, Sebit B, Lual MY, Baba SP, Chanda E. Malaria control in South Sudan, 2006-2013: strategies, progress and challenges. Malar J[Internet]. 2013 Oct 27[2022 Dec 26];12(1):374. https://doi.org/10.1186/1475-2875-12-374 PubMed | Google Scholar
- Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF, Aboyans V, Adetokunboh O, Afshin A, Agrawal A, Ahmadi A, Ahmed MB, Aichour AN, Aichour MTE, Aichour I, Aiyar S, Alahdab F, Al-Aly Z, Alam K, Alam N, Alam T, Alene KA, Al-Eyadhy A, Ali SD, Alizadeh-Navaei R, Alkaabi JM, Alkerwi A, Alla F, Allebeck P, Allen C, Al-Raddadi R, Alsharif U, Altirkawi KA, Alvis-Guzman N, Amare AT, Amini E, Ammar W, Amoako YA, Anber N, Andersen HH, Andrei CL, Androudi S, Ansari H, Antonio CAT, Anwari P, Ärnlöv J, Arora M, Artaman A, Aryal KK, Asayesh H, Asgedom SW, Atey TM, Avila-Burgos L, Avokpaho EFG, Awasthi A, Babalola TK, Bacha U, Balakrishnan K, Barac A, Barboza MA, Barker-Collo SL, Barquera S, Barregard L, Barrero LH, Baune BT, Bedi N, Beghi E, Béjot Y, Bekele BB, Bell ML, Bennett JR, Bensenor IM, Berhane A, Bernabé E, Betsu BD, Beuran M, Bhatt S, Biadgilign S, Bienhoff K, Bikbov B, Bisanzio D, Bourne RRA, Breitborde NJK, Bulto LNB, Bumgarner BR, Butt ZA, Cahuana-Hurtado L, Cameron E, Campuzano JC, Car J, Cárdenas R, Carrero JJ, Carter A, Casey DC, Castañeda-Orjuela CA, Catalá-López F, Charlson FJ, Chibueze CE, Chimed-Ochir O, Chisumpa VH, Chitheer AA, Christopher DJ, Ciobanu LG, Cirillo M, Cohen AJ, Colombara D, Cooper C, Cowie BC, Criqui MH, Dandona L, Dandona R, Dargan PI, das Neves J, Davitoiu DV, Davletov K, de Courten B, Defo BK, Degenhardt L, Deiparine S, Deribe K, Deribew A, Dey S, Dicker D, Ding EL, Djalalinia S, Do HP, Doku DT, Douwes-Schultz D, Driscoll TR, Dubey M, Duncan BB, Echko M, El-Khatib ZZ, Ellingsen CL, Enayati A, Ermakov SP, Erskine HE, Eskandarieh S, Esteghamati A, Estep K, Farinha CS e S, Faro A, Farzadfar F, Feigin VL, Fereshtehnejad SM, Fernandes JC, Ferrari AJ, Feyissa TR, Filip I, Finegold S, Fischer F, Fitzmaurice C, Flaxman AD, Foigt N, Frank T, Fraser M, Fullman N, Fürst T, Furtado JM, Gakidou E, Garcia-Basteiro AL, Gebre T, Gebregergs GB, Gebrehiwot TT, Gebremichael DY, Geleijnse JM, Genova-Maleras R, Gesesew HA, Gething PW, Gillum RF, Giref AZ, Giroud M, Giussani G, Godwin WW, Gold AL, Goldberg EM, Gona PN, Gopalani SV, Gouda HN, Goulart AC, Griswold M, Gupta R, Gupta T, Gupta V, Gupta PC, Haagsma JA, Hafezi-Nejad N, Hailu AD, Hailu GB, Hamadeh RR, Hambisa MT, Hamidi S, Hammami M, Hancock J, Handal AJ, Hankey GJ, Hao Y, Harb HL, Hareri HA, Hassanvand MS, Havmoeller R, Hay SI, He F, Hedayati MT, Henry NJ, Heredia-Pi IB, Herteliu C, Hoek HW, Horino M, Horita N, Hosgood HD, Hostiuc S, Hotez PJ, Hoy DG, Huynh C, Iburg KM, Ikeda C, Ileanu BV, Irenso AA, Irvine CMS, Islam SMS, Jacobsen KH, Jahanmehr N, Jakovljevic MB, Javanbakht M, Jayaraman SP, Jeemon P, Jha V, John D, Johnson CO, Johnson SC, Jonas JB, Jürisson M, Kabir Z, Kadel R, Kahsay A, Kamal R, Karch A, Karimi SM, Karimkhani C, Kasaeian A, Kassaw NA, Kassebaum NJ, Katikireddi SV, Kawakami N, Keiyoro PN, Kemmer L, Kesavachandran CN, Khader YS, Khan EA, Khang YH, Khoja ATA, Khosravi MH, Khosravi A, Khubchandani J, Kiadaliri AA, Kieling C, Kievlan D, Kim YJ, Kim D, Kimokoti RW, Kinfu Y, Kissoon N, Kivimaki M, Knudsen AK, Kopec JA, Kosen S, Koul PA, Koyanagi A, Kulikoff XR, Kumar GA, Kumar P, Kutz M, Kyu HH, Lal DK, Lalloo R, Lambert TLN, Lan Q, Lansingh VC, Larsson A, Lee PH, Leigh J, Leung J, Levi M, Li Y, Li Kappe D, Liang X, Liben ML, Lim SS, Liu PY, Liu A, Liu Y, Lodha R, Logroscino G, Lorkowski S, Lotufo PA, Lozano R, Lucas TCD, Ma S, Macarayan ERK, Maddison ER, Magdy Abd El Razek M, Majdan M, Majdzadeh R, Majeed A, Malekzadeh R, Malhotra R, Malta DC, Manguerra H, Manyazewal T, Mapoma CC, Marczak LB, Markos D, Martinez-Raga J, Martins-Melo FR, Martopullo I, McAlinden C, McGaughey M, McGrath JJ, Mehata S, Meier T, Meles KG, Memiah P, Memish ZA, Mengesha MM, Mengistu DT, Menota BG, Mensah GA, Meretoja TJ, Meretoja A, Millear A, Miller TR, Minnig S, Mirarefin M, Mirrakhimov EM, Misganaw A, Mishra SR, Mohamed IA, Mohammad KA, Mohammadi A, Mohammed S, Mokdad AH, Mola GLD, Mollenkopf SK, Molokhia M, Monasta L, Montañez JC, Montico M, Mooney MD, Moradi-Lakeh M, Moraga P, Morawska L, Morozoff C, Morrison SD, Mountjoy-Venning C, Mruts KB, Muller K, Murthy GVS, Musa KI, Nachega JB, Naheed A, Naldi L, Nangia V, Nascimento BR, Nasher JT, Natarajan G, Negoi I, Ngunjiri JW, Nguyen CT, Nguyen QL, Nguyen TH, Nguyen G, Nguyen M, Nichols E, Ningrum DNA, Nong VM, Noubiap JJN, Ogbo FA, Oh IH, Okoro A, Olagunju AT, Olsen HE, Olusanya BO, Olusanya JO, Ong K, Opio JN, Oren E, Ortiz A, Osman M, Ota E, Pa M, Pacella RE, Pakhale S, Pana A, Panda BK, Panda-Jonas S, Papachristou C, Park EK, Patten SB, Patton GC, Paudel D, Paulson K, Pereira DM, Perez-Ruiz F, Perico N, Pervaiz A, Petzold M, Phillips MR, Pigott DM, Pinho C, Plass D, Pletcher MA, Polinder S, Postma MJ, Pourmalek F, Purcell C, Qorbani M, Quintanilla BPA, Radfar A, Rafay A, Rahimi-Movaghar V, Rahman MHU, Rahman M, Rai RK, Ranabhat CL, Rankin Z, Rao PC, Rath GK, Rawaf S, Ray SE, Rehm J, Reiner RC, Reitsma MB, Remuzzi G, Rezaei S, Rezai MS, Rokni MB, Ronfani L, Roshandel G, Roth GA, Rothenbacher D, Ruhago GM, Sa R, Saadat S, Sachdev PS, Sadat N, Safdarian M, Safi S, Safiri S, Sagar R, Sahathevan R, Salama J, Salamati P, Salomon JA, Samy AM, Sanabria JR, Sanchez-Niño MD, Santomauro D, Santos IS, Santric Milicevic MM, Sartorius B, Satpathy M, Schmidt MI, Schneider IJC, Schulhofer-Wohl S, Schutte AE, Schwebel DC, Schwendicke F, Sepanlou SG, Servan-Mori EE, Shackelford KA, Shahraz S, Shaikh MA, Shamsipour M, Shamsizadeh M, Sharma J, Sharma R, She J, Sheikhbahaei S, Shey M, Shi P, Shields C, Shigematsu M, Shiri R, Shirude S, Shiue I, Shoman H, Shrime MG, Sigfusdottir ID, Silpakit N, Silva JP, Singh JA, Singh A, Skiadaresi E, Sligar A, Smith DL, Smith A, Smith M, Sobaih BHA, Soneji S, Sorensen RJD, Soriano JB, Sreeramareddy CT, Srinivasan V, Stanaway JD, Stathopoulou V, Steel N, Stein DJ, Steiner C, Steinke S, Stokes MA, Strong M, Strub B, Subart M, Sufiyan MB, Sunguya BF, Sur PJ, Swaminathan S, Sykes BL, Tabarés-Seisdedos R, Tadakamadla SK, Takahashi K, Takala JS, Talongwa RT, Tarawneh MR, Tavakkoli M, Taveira N, Tegegne TK, Tehrani-Banihashemi A, Temsah MH, Terkawi AS, Thakur JS, Thamsuwan O, Thankappan KR, Thomas KE, Thompson AH, Thomson AJ, Thrift AG, Tobe-Gai R, Topor-Madry R, Torre A, Tortajada M, Towbin JA, Tran BX, Troeger C, Truelsen T, Tsoi D, Tuzcu EM, Tyrovolas S, Ukwaja KN, Undurraga EA, Updike R, Uthman OA, Uzochukwu BSC, van Boven JFM, Vasankari T, Venketasubramanian N, Violante FS, Vlassov VV, Vollset SE, Vos T, Wakayo T, Wallin MT, Wang YP, Weiderpass E, Weintraub RG, Weiss DJ, Werdecker A, Westerman R, Whetter B, Whiteford HA, Wijeratne T, Wiysonge CS, Woldeyes BG, Wolfe CDA, Woodbrook R, Workicho A, Xavier D, Xiao Q, Xu G, Yaghoubi M, Yakob B, Yano Y, Yaseri M, Yimam HH, Yonemoto N, Yoon SJ, Yotebieng M, Younis MZ, Zaidi Z, Zaki MES, Zegeye EA, Zenebe ZM, Zerfu TA, Zhang AL, Zhang X, Zipkin B, Zodpey S, Lopez AD, Murray CJL. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet[Internet]. 2017 Sep 16[cited 2023 Jan 30];390(10100):1151-210. https://doi.org/10.1016/S0140-6736(17)32152-9 Google Scholar
- Mercado CE, Ekapirat N, Dondorp AM, Maude RJ. An assessment of national surveillance systems for malaria elimination in the Asia Pacific. Malar J[Internet]. 2017 Mar 21[cited 2022 Dec 26];16(1):127. https://doi.org/10.1186/s12936-017-1774-3 PubMed | Google Scholar
- Canavati SE, Lawpoolsri S, Quintero CE, Nguon C, Ly P, Pukrittayakamee S, Sintasath D, Singhasivanon P, Peeters Grietens K, Whittaker MA. Village malaria worker performance key to the elimination of artemisinin-resistant malaria: a Western Cambodia health system assessment. Malar J[Internet]. 2016 May 20[cited 2022 Dec 26];15(1):282. https://doi.org/10.1186/s12936-016-1322-6 PubMed | Google Scholar
- Ibrahim BS, Abubakar AA, Bajoga UA, Nguku PM. Evaluation of the malaria surveillance system in Kaduna state, Nigeria 2016. Online Journal of Public Health Informatics[Internet]. 2017 May 2[cited 2022 Dec 26];9(1). https://doi.org/10.5210/ojphi.v9i1.7775 . Google Scholar
- Beck HE, Zimmermann NE, McVicar TR, Vergopolan N, Berg A, Wood EF. Present and future köppen-geiger climate classification maps at 1-km resolution. Sci Data[Internet]. 2018 Oct 30[cited 2023 Jan 30];5(1):180214. https://doi.org/10.1038/sdata.2018.214 . Google Scholar
- Abubakar IR. Abuja city profile. Cities[Internet]. 2014 Dec 1[cited 2022 Dec 26];41:81-91. https://doi.org/10.1016/j.cities.2014.05.008 Google Scholar
- Charan J, Biswas T. How to calculate sample size for different study designs in medical research? Indian J Psychol Med[Internet]. 2013 Apr[cited 2023 Jan 30];35(2):121-6. https://doi.org/10.4103/0253-7176.116232 Google Scholar
- Fox, N, Hunn, A, & Mathers, N. Sampling and Sample Size Calculation[Internet]. The NIHR Research Design Service for the East Midlands; 2009[cited 2023 Jan 30].
- World Health Organization. Guidelines for the treatment of Malaria - 3rd Edition[Internet]. WHO;[cited 2023 Jan 2]. 313p. Google Scholar
- Ruebush TK, Weller SC, Klein RE. Qualities of an ideal volunteer community malaria worker: A comparison of the opinions of community residents and national malaria service staff. Soc Sci Med[Internet]. 1994 Jul 1;39(1):123-31. https://doi.org/10.1016/0277-9536(94)90172-4 . Google Scholar
- Panday S, Bissell P, Teijlingen EV, Simkhada P. Perceived barriers to accessing Female Community Health Volunteers' (FCHV) services among ethnic minority women in Nepal: A qualitative study. PLoS One[Internet]. 2019 Jun 10[cited 2023 Jan 30];14(6):e0217070. https://doi.org/10.1371/journal.pone.0217070 PubMed | Google Scholar
- Visa TI, Ajumobi O, Bamgboye E, Ajayi I, Nguku P. Evaluation of malaria surveillance system in Kano State, Nigeria, 2013-2016. Infect Dis Poverty[Internet]. 2020 Feb 10[cited 2023 Jan 30];9(1):15. https://doi.org/10.1186/s40249-020-0629-2 PubMed | Google Scholar
- Olugbade OT, Ladipo TO, Isreal O, Adedire EO, Adedokun B, Ajumobi O, Olayinka A, Ajayi I. Malaria surveillance system evaluation, Oyo state, Nigeria 2012. Int J Infect Dis [Internet]. 2014 Apr 1[cited 2022 Dec 30];21:275-6. https://doi.org/10.1016/j.ijid.2014.03.992 . Google Scholar
-
Abimbola S, Olanipekun T, Igbokwe U, Negin J, Jan S, Martiniuk A, Ihebuzor N, Aina M. How decentralisation influences the retention of primary health care workers in rural Nigeria. Glob Health Action[Internet]. 2015 Dec[cited 2023 Jan 30];8(1):26616. https://doi.org/10.3402/gha.v8.26616 . PubMed | Google Scholar
- Frumence G, Nyamhanga T, Mwangu M, Hurtig AK. Challenges to the implementation of health sector decentralization in Tanzania: experiences from Kongwa district council. Glob Health Action[Internet]. 2013 Aug 29[cited 2022 Dec 26]; 6(1):20983. https://doi.org/10.3402/gha.v6i0.20983 PubMed | Google Scholar
- Aniwada EC, Obionu CN. Disease surveillance and notification, knowledge and practice among private and public primary health care workers in Enugu State, Nigeria: A comparative study. Br J Med Med Res[Internet]. 2016 Jan 10[cited 2022 Dec 26];13(3):1-10. https://doi.org/10.9734/BJMMR/2016/23249 Google Scholar
- Fernando SD, Ainan S, Premaratne RG, Rodrigo C, Jayanetti SR, Rajapakse S. Challenges to malaria surveillance following elimination of indigenous transmission: findings from a hospital-based study in rural Sri Lanka. International health[Internet]. 2015 Sep 1[cited 2023 Jan 30];7(5):317-23. https://doi.org/10.1093/inthealth/ihv046 Google Scholar
- Ladi-Akinyemi T, Amoran O, Ogunyemi A, Kanma-Okafor O, Onajole A. Knowledge and implementation of the National Malaria Control Programme among health-care workers in primary health-care centers in Ogun State, Nigeria. J Clin Sci[Internet]. 2018 Feb 23[cited 2023 Jan 30];15(1):48-54. https://doi.org/10.4103/jcls.jcls_55_17 Google Scholar
- Avong YK, Jatau B, Gurumnaan R, Danat N, Okuma J, Usman I, Mordi D, Ukpabi B, Kayode GA, Dutt S, El-Tayeb O, Afolabi B, Ambrose I, Agbaji O, Osakwe A, Ibrahim A, Ogar C, Nosiri H, Avong EB, Adekanmbi V, Uthman O, Abimiku A, Oni YO, Mensah CO, Dakum P, Mberu KE, Ogundahunsi OAT. Addressing the under-reporting of adverse drug reactions in public health programs controlling HIV/AIDS, Tuberculosis and Malaria: A prospective cohort study. PLoS One[Internet]. 2018 Aug 22[cited 2022 Dec 26];13(8):e0200810. https://doi.org/10.1371/journal.pone.0200810 PubMed | Google Scholar
- Ofili AN, Ugwu EN, Ziregbe A, Richards R, Salami S. Knowledge of disease notification among doctors in government hospitals in Benin Ciy, Edo State, Nigeria. Public Health[Internet]. 2017 May 1[cited 2022 Dec 26];117(3):214-7. https://doi.org/10.1016/S0033-3506(02)00021-5 . Google Scholar
- Ohrt C, Roberts KW, Sturrock HJW, Wegbreit J, Lee BY, Gosling RD. Information systems to support surveillance for malaria elimination. Am J Trop Med Hyg[Internet]. 2015 Jul[cited 2022 Dec 26];93(1):145-152. https://doi.org/10.4269/ajtmh.14-0257 PubMed | Google Scholar
- Alwan K, Awoke T, Tilahun B. Knowledge and Utilization of Computers Among Health Professionals in a Developing Country: A Cross-Sectional Study. JMIR Hum Factors[Internet]. 2015 Mar 26[cited 2022 Dec 26];2(1):e4. https://doi.org/10.2196/humanfactors.4184 PubMed | Google Scholar
- Tate KE, Gardner RM. Computers, quality, and the clinical laboratory: a look at critical value reporting. Proc Annu Symp Comput Appl Med Care. 1993:193-7. PubMed | Google Scholar
- Nghochuzie NN, Olwal CO, Udoakang AJ, Amenga-Etego LN, Amambua-Ngwa A. Pausing the Fight Against Malaria to Combat the COVID-19 Pandemic in Africa: Is the Future of Malaria Bleak? Front Microbiol[Internet]. 2020 Jun 18[cited 2022 Dec 26];11:1476. https://doi.org/10.3389/fmicb.2020.01476 PubMed | Google Scholar
- Rogerson SJ, Beeson JG, Laman M, Poespoprodjo JR, William T, Simpson JA, Price RN, ACREME Investigators. Identifying and combating the impacts of COVID-19 on malaria. BMC Med[Internet]. 2020 Jul 30[cited 2022 Dec 26];18(1):239. https://doi.org/10.1186/s12916-020-01710-x PubMed | Google Scholar
- Rumisha SF, Smith T, Abdulla S, Masanja H, Vounatsou P. Modelling heterogeneity in malaria transmission using large sparse spatio-temporal entomological data. Glob Health Action[Internet]. 2014 Jun 24[cited 2022 Dec 26];7(1):22682. https://doi.org/10.3402/gha.v7.22682 PubMed | Google Scholar
- Chanda E, Mukonka VM, Kamuliwo M, Macdonald MB, Haque U. Operational scale entomological intervention for malaria control: strategies, achievements and challenges in Zambia. Malar J[Internet]. 2013 Jan 8[cited 2022 Dec 26];12(1):10. https://doi.org/10.1186/1475-2875-12-10 PubMed | Google Scholar
- Kyabayinze DJ, Achan J, Nakanjako D, Mpeka B, Mawejje H, Mugizi R, Kalyango JN, D'Alessandro U, Talisuna A, Jean-Pierre Vg. Parasite-based malaria diagnosis: are health systems in Uganda equipped enough to implement the policy? BMC Public Health[Internet]. 2012 Aug 24[cited 2022 Dec 26];12(1):695. https://doi.org/10.1186/1471-2458-12-695 PubMed | Google Scholar
- Abreha T, Alemayehu B, Tadesse Y, Gebresillassie S, Tadesse A, Demeke L, Zewde F, Habtamu M, Tadesse M, Yadeta D, Teshome D, Mekasha A, Gobena K, Bogale H, Melaku Z, Reithinger R, Teka H. Malaria diagnostic capacity in health facilities in Ethiopia. Malar J[Internet]. 2014 Jul 29[cited 2022 Dec 26];13:292. https://doi.org/10.1186/1475-2875-13-292 PubMed | Google Scholar
- Wilson ML. Laboratory diagnosis of Malaria: Conventional and rapid diagnostic methods. Arch Pathol Lab Med[Internet]. 2013 Jun 1[cited 2022 Dec 26];137(6):805-11. https://doi.org/10.5858/arpa.2011-0602-RA. Google Scholar
- Jaleta F, Garoma G, Gerenfes T. Evaluation Of Malaria Microscopy Diagnosis Performance In Public Hospitals Of Eastern And Centeral Part Of Oromia Region, Ethiopia, 2019. Research Square [Preprint] 2020 [posted 2020 April 14; cited 2022 Dec 26]: [14 p.]. https://doi.org/10.21203/rs.3.rs-21700/v1 Google Scholar
- Bamiselu OF, Ajayi I, Fawole O, Dairo D, Ajumobi O, Oladimeji A, Steven Y. Adherence to malaria diagnosis and treatment guidelines among healthcare workers in Ogun State, Nigeria. BMC Public Health[Internet]. 2016 Aug 19[cited 2022 Dec 26];16(1):828. https://doi.org/10.1186/s12889-016-3495-x PubMed | Google Scholar
- Abdulraheem SI, Olapipo AR, Amodu MO. Primary health care services in Nigeria: Critical issues and strategies for enhancing the use by the rural communities. J Public Heal Epidemiol[Internet]. 2012 Jan 31[cited 2022 Dec 26];4(1):5-13. https://doi.org/10.5897/JPHE11.133 Google Scholar
- Alenoghena I, Aigbiremolen A, Abejegah C, Eboreime E. Primary Health Care in Nigeria: Strategies and constraints in implementation. Int J Community Res[Internet]. 2014 Sep 12[cited 2022 Dec 26];3(3):74-9. Google Scholar




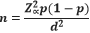
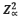 = Standard normal deviate. (At 5% type 1 error where (p<0.05), standard normal deviate is 1.96
= Standard normal deviate. (At 5% type 1 error where (p<0.05), standard normal deviate is 1.96
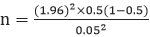 = 384
= 384
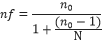
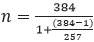 = 155
= 155