HIV-Malaria co-infection and its determinants among patients attending antiretroviral treatment clinic in Zaria, Kaduna state, Nigeria
Shafi'u Dahiru Gumel1,4,8,&, Abdulrasul Ibrahim2, Adebola Tolulope Olayinka3, Muhammed Sani Ibrahim4, Muhammad Shakir Balogun1, Afara Dahiru5, Ikeoluwapo Ajayi1,6, Olufemi Ajumobi1, Isiyaku Ahmadu7, Abubakar Song7, Asma´u Ibrahim Maifada7, Habibu Abdullahi7
1Nigeria Field Epidemiology and Laboratory Training Program, Abuja, Nigeria, 2Department of Medical Microbiology, Ahmadu Bello Teaching Hospital, Zaria, Kaduna State, Nigeria, 3Nigeria Centre for Disease Control, Abuja, Nigeria, 4Department of Community Medicine, Faculty of Medicine, Ahmadu Bello University Zaria, Kaduna State, Nigeria, 5Department of Nursing, College of Nursing and Midwifery Hadejia, Jigawa State, Nigeria, 6Department of Epidemiology and Medical Statistics, Faculty of Public Health, College of Medicine, University of Ibadan, Oyo State, Nigeria, 7National Tuberculosis and Leprosy Treatment Centre, Saye, Kaduna State, Nigeria, 8Department of Public Health, State Ministry of Health, Jigawa State, Nigeria.
&Corresponding author
Shafi´u Dahiru Gumel, Nigeria Field Epidemiology and Laboratory Training Program, Abuja, Nigeria shafiudahiru@yahoo.com
Introduction:
Malaria and HIV are two important global public health problems. Together, they cause more than two million deaths each year. In sub-Saharan Africa alone, more than 29 million people are living with HIV/AIDS and about 70% of population is at risk to malaria infection. Nigeria accounts for about a quarter of the global malaria cases and tenth of the global HIV cases. Recent theories suggested possibilities of high occurrence of HIV-malaria co-infection wherever there is geographical overlap of the two diseases. We therefore conducted this study to determine the prevalence of HIV-malaria co-infection and its determinants in a malaria endemic setting.
Methods:
We conducted a cross-sectional study. Two hundred and sixty-two clients attending antiretroviral treatment (ART) clinic in Zaria, Kaduna State were enrolled between February and April 2018 using systematic sampling technique. Questionnaires were administered to collect information on respondents� personal characteristics and to assess their knowledge, perception and practices on malaria prevention. Venous blood samples were collected and analyzed for malaria parasite, viral load, CD4, and FBC using Giemsa stained light microscopy, COBAS TaqMan equipment, BD FACS� flow cytometer, and Sysmex haematology analyser respectively. Descriptive and inferential statistics were performed, predictors of HIV-malaria co-infection were ascertained at multivariate analysis.
Results:
Median age of the respondents was 33 years, 52% were females, 65% were married, 65% were employed, 57% lived in urban residence, and 34% had tertiary education. The prevalence of malaria co-infection among HIV patients was found to be 22.9%. Significant risk factors for the co-infection were high HIV viral load (aOR= 3.30, C.I = 1.15-9.45), being co-infected with TB (aOR= 5.60, C.I = 1.34-23.33), poor knowledge of malaria infection (aOR= 3.12, C.I = 1.27-7.72) and poor practice of malaria prevention (aOR= 13.30, C.I = 4.88-36.23).
Conclusion:
The level of occurrence of malaria among HIV infected patients in this setting calls for attention. We recommended that health education on malaria should be a priority in malaria control programme; the programmes for control of HIV, malaria and TB should collaborate to ensure integrated service delivery and that people living with HIV/AIDS should be given special consideration for malaria prevention.
Introduction

Human Immunodeficiency Virus (HIV) and malaria are major public health problems which are endemic in Nigeria [1,2]. The HIV infection weakens the human immune system [3] making an individual more prone to other infections like malaria [4,5]. Malaria on the other hand is caused by Plasmodium, a single cell microorganism that invades red blood cells, with greater propensity for severity and death in the presence of immunosuppression [5]. It has also been reported that co-infection of HIV with malaria increases morbidity and mortality from HIV [6].
Risk of contracting malaria and developing severe disease varies between individuals and populations [
7]. These include children under 5 years of age [
8], pregnant women [
4], and immune-compromised patients such as those with HIV/AIDS [
6]. According to the World Health Organization (WHO), malaria and HIV combined, cause more than two million deaths each year [
4]. This is more so when the two diseases bring about common consequences such as anaemia [
5,
9-11].
There were an estimated 36.9 million people living with HIV (PLWHIV) in 2017 [
12]. Sub-Saharan Africa is the most affected region contributing about 66% (25.8 million) and Nigeria had 3.1 million people living with HIV in 2017 [
13].
In 2017, 219 million malaria cases and 435, 000 malaria related deaths were reported globally [
14]. Nigeria contributes about 23% of the global malaria cases and around 250,000 Nigerian children die from malaria every year [
15]. It is also estimated that 97% of Nigerian population is at risk of malarial infection [
15].
There are reports that HIV and malaria co-infection may occur in any area that has high prevalence of the two infections [
16,
17] and that much possibilities of deleterious interaction between the two diseases exist in the co-infected patients [
5,
6,
16]. Some complications, such as anaemia, which are common to both HIV and malaria are also likely to be worse with the co-infection [
18]. In co-infected individuals, HIV - malaria interactions adversely affect the outcome of both conditions [
19] especially in pregnant women and infants born to HIV infected mothers [
20].
It has also been indicated that anti-retroviral drugs interact with both new and established anti-malaria drugs, complicating treatment efforts for both infections [
21]. On the other hand, some of these drugs used in highly active antiretroviral therapy (HAART) are shown to have antimalarial action [
19] in which inhibiting growth of malarial parasite and Pharmacokinetic interactions of HAART drugs with antimalarials mostly involve protease inhibitors (PIs) and non-nucleoside reverse-transcriptase inhibitor (NNRTIs) [
22,
23].
Nigerian government has instituted several interventions for malaria control including 1) Prevention of malaria transmission through vector control as part of an Integrated Vector Management (IVM) strategy mainly through the promotion of insecticide-treated mosquito nets (ITNs) and indoor residual spraying; 2) Prevention and treatment of malaria in pregnancy through intermittent preventive treatment (IPT), and 3) Prompt diagnosis and adequate treatment of clinical cases [
24]. Studies on prophylaxis with co-trimaxazole in HIV-infected patients show significant protection against malaria [
25]. It is however advised that people who have HIV-AIDS and uncomplicated P. falcifarum malaria to avoid artesunate + Sulfadoxine-pyrimethamine (SP) if they are also receiving co-trimoxazole, and avoid artesunate + amodiaquine if they are also using efavirenz or zidovudine [
25].
This study was designed to determine the prevalence of malaria parasitaemia among HIV-positive clients in malaria endemic setting and to identify the determinants of this co-infection.
Methods


Study area
This study was conducted in Kaduna State, north-west Nigeria. In 2016, Kaduna state with HIV prevalence of 9.2% was ranked 3
rdamong states with highest HIV burden in Nigeria [
26]. There are over 1,000 primary healthcare (PHC) facilities and forty-two HIV treatment centers in the state. The health-care institution used (NTBLTC) was purposively selected for the study because of its strategic functions and location: (a) referral centre for HIV positive patients, (b) Research Centre with well-equipped laboratory, and (c) located in the area with high HIV prevalence (Kaduna State).
The HIV clinic at the National Tuberculosis and Leprosy Treatment Centre (NTBLTC) provides, alongside patient care, a comprehensive health education to the patients. This helps patient with knowledge about HIV and malaria infections, including prevention, treatment, and other aspect of care. It also equipped patients with tools that will enable them to participate more actively in decisions regarding their medical care.
The area has two seasons, the rainy season (April-November) and the dry season (December-March)[
27]. Malaria is endemic in the state, but transmission is usually higher towards the end of the rainy season[
2].
Study design
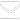
A cross-sectional study was conducted among 262 consented persons living with HIV/AIDS (PLHIV) receiving treatment at the National Tuberculosis and Leprosy Training Center, Saye, Zaria, Kaduna State. The minimum sample size of 262 for the study was determined using
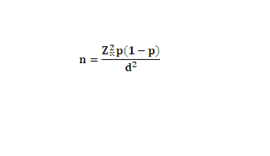
[
28];
taking prevalence (p) of HIV-malaria co-infection = 21% [
29], confidence interval 95% and margin of error as 5%. Systematic sampling technique was used at a sampling interval of 16 (4,350/262) in a sampling frame of 4,350 hospital records. We included patients who had not received any anti-malaria drugs for one-month period, had tested positive for HIV, and who were neither severely ill nor mentally retarded.
Data collection
Structured, interviewer administered questionnaire was used to collect data for this study. The questionnaire had five sections namely, Section A: Demographic information, Section B: Clinical information, Section C: Knowledge of malaria infection, Section D: Perception on malaria infection, and Section E: Practice of malaria prevention and control. The questionnaire was developed from earlier studies related to HIV and malaria[
30-33]; it was pre-tested on 30 volunteers attending HIV clinic at Gumel general hospital Jigawa state. Trained research assistants were used to administer the questionnaires.
The questionnaire had twelve questions on knowledge of malaria infection, six on perception on malaria infection and eight on practices of malaria prevention and control. Grading of these three parameters was done by assigning one mark for each correct answer and for multiple choices each correct response has one mark. A total of fifty, six, and sixteen marks were respectively allocated to knowledge, perception and practices. Fifty percent was considered as the cut-off point; in each category therefore, ≥ 50% marks was considered as good and < 50% marks as poor. Viral load and CD4 count data were grouped into two, "high" and "low" each. A high viral load is any viral load result > 1000 copies/ml[
34]; while a low CD4 count is any CD4 count ≤ 350cells/µl [
35].
Laboratory Examination
Two EDTA vacutainer tubes were used to collect 15 ml blood sample from each respondent; 10 ml and 5 ml separately for HIV viral load and the remaining laboratory tests respectively. From the 5 ml sample, thick and thin blood smears were made on grease-free slides and stained with Giemsa to determine species of malaria parasites and parasite density according to the earlier published protocol [
36]. Parasite densities were estimated by counting the number of malaria parasites against leukocytes using the following formula:

[
37].
All stained slides were examined by microscopy and read by two competent microscopists using 100 power fields under oil immersion. Polymerase Chain Reaction (PCR) using COBAS TaqMan equipment was employed to estimate the viral load of each respondent, and BD FACSPresto™ machine was used to estimate the CD4 count of each respondent adhering to the equipment manufacturer instructions. This study used the TB status of patients as indicated in their file which in turn was determined by PCR method.
Statistical analysis
All data were entered into Microsoft excel, cleaned and imported into Epi Info version 7 statistical software for analysis. Univariate analysis including descriptive statistics like percentages and frequencies of the distribution of all variables of interest was performed. Chi-square test was conducted to determine the association between categorical variables i.e. association between HIV and malaria co-infection status in relation to age group, gender, place of residence, education level, employment status, ARV regimen, treatment duration, viral load level, CD
4count, TB status and knowledge, perception and practice of malaria infection prevention. Logistic regression model was conducted. Odds-ratios (OR) and adjusted odds-ratios (aOR) with 95% confidence interval (CI) were used to measure the strength of associations. All tests were two-tailed and P value < 0.05 was considered statistically significant.
Ethical consideration
Ethical approval for the study (Ref: NTBL/TRG/ZA/20/Vol I) was obtained from the Research and Ethics Committee of NTBLTC, Zaria. Written informed consent was obtained from the respondents above the age of 16 years. For participants under 16 years old, written informed consent was obtained from their parent or guardian.
Results


Two hundred and sixty-two subjects were enrolled in this study, the median age of the respondents was 32.50 years (Range: 3-74 years). One hundred and thirty-six respondents (51.9%) were females, 170 (64.9%) were married, 149 (56.9%) lived in urban residence, 89 (34.0%) had tertiary education, 115 (43.9%) were formally employed and 134 (51.2%) were of the Hausa-Fulani ethnic group (Table1).
The prevalence of malaria co-infection among HIV patients was found to be 22.9% (17.95% – 28.47%, 95% C.L). On bivariate analysis, there was statistically significant association between malaria parasitaemia and place of residence, educational status, occupation, and knowledge of malaria infection, and practice of malaria infection prevention and control among the respondents (
Table 1). Similarly, viral load, CD4 count, and TB status were significantly associated with malaria parasitaemia of the respondents (
Table 2). However, on multivariate analysis only viral load, TB status, knowledge of malaria infection, and practice of malaria infection prevention and control were independently associated with malaria parasitaemia (
Table 3).
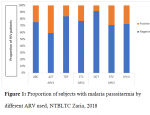
All the subjects were using ARV combination that consists of two nucleoside reverse-transcriptase inhibitors (NRTIs) plus a non-nucleoside reverse-transcriptase inhibitor (NNRTI) or an integrase inhibitor (INSTI). The ARV drugs used by the subjects were abacavir (ABC), zidovudine (AZT), efavirenz (EFV), lamivudine (3TC), dolutegravir (DGT), tenofovir (TDF), and lopinavir/ritonavir (LPV/r) in various combinations. The proportion of subjects that has malaria parasitaemia was higher among those using AZT and lower among those using DGT (
Figure 1).
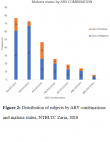
Seventy seven subjects (29.4%) were using TDF/3TC/EFV combination; 73 (27.9%) were using TDF/3TC/DGT combination; 47 (17.9%) were using AZT/3TC/EFV combination; 26 (9.9%) were using TDF/3TC/LPV/r combination; 19 (7.3%) were using AZT/3TC/LPV/r combination; 14 (5.3%) were using ABC/3TC/EFV combination; and 6 (2.3%) were using ABC/3TC/LPV/r combination (
Figure 2). Proportion of those that have malaria parasitaemia is higher among those on AZT/3TC/EFV combination (44.7%), and lower among those on TDF/3TC/DGT (8.2%) combination (
Figure 2).
Mean parasite density of the subjects was 1781.5 ± 3963. Student t-test was used to compare between some immunological parameters of the subjects that were malaria positive and that of those that were malaria negative. Mean log viral load of those that were malaria positive (4.51 ± 0.75) was significantly higher than that of those that were malaria negative (3.84 ± 0.85). On the other hand, CD4 count was significantly lower in malaria positive subjects compared to malaria negative ones (p<0.05) (
Table 4).
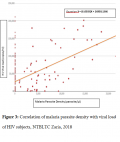
Among those that were malaria positive, parasite density was found to be positively correlated with HIV viral load (p<0.05), and the equation for the association was Y = (6.05)X + 26958.14 (
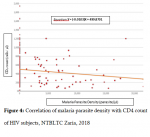
Figure 3). On the other hand, the parasite density was found to be negatively correlated with CD
4count (p<0.05), equation for the association was Y = (−0.0103)X ÷ 499.87 (
Figure 4).
Discussion


In this study, the prevalence of malaria co-infection among individuals with HIV infection is 22.9%; this was slightly higher than 18.9% reported from Anambra, southeastern Nigeria[38] and 21% from Jos, northcentral Nigeria[29]. The slight difference could be due to differing malaria burden between these 3 geo-political zones of the country. The proportion of HIV patients reported to be co-infected with malaria by the present and other studies indicates high occurrences of this phenomenon. In an attempt to explain the mechanism, it was indicated in 2014 that opsonizing antibodies and phagocytosis were significantly reduced in HIV-infected individuals[39]; and these processes are very vital in body´s fight against malaria.
In this study, the prevalence of the co-infection does not differ significantly between males and females and is not associated with age. This agrees with findings from a study in Kano, North-West Nigeria[
40] but contrary to another one from Jos North-Central Nigeria[
29]. This could be explained by the fact that the study conducted in Jos was among subjects that were mainly urban residents unlike that of the present study which comprises both urban and rural dwellers. In urban areas, there seems to be some differences in knowledge and practice of malaria infection prevention between the different gender and age groups unlike in rural areas where everything seems to be the same, this may explain the disparity in findings of these studies.
Risk factors for HIV-malaria co-infection were also studied in this study; viral load, TB status, knowledge of malaria infection, and practice of malaria infection prevention were found to be associated with malaria co-infection in HIV patients. Other factors that may confound or modify the association were place of residence, educational status, and occupation.
Our study found that malaria co-infected HIV subjects have higher levels of HIV viral load and that malaria parasite density increases with increase viral load. This may suggest possible interaction between malaria parasite and the virus. In our study also, parasite density increases with decrease in CD4 count. These findings agree with several similar studies and theories. For instance, Kwenti in his systematic review in 2018 indicated that malaria infection is associated with an increase in the HIV viral load in vivo and in vitro, and HIV infection, in turn, is associated with an increased malaria parasite density[
17]. Even though people residing in areas of stable malaria transmission develop humoral and cell-mediated immunity to malaria parasite; it was shown that this immunity can be altered in HIV-infected persons and could influence the frequency and course of malaria infection[
41]. This could therefore explain the association of the viral load with malaria infection where by the risk increases with increase in viral load.
With regards to TB, it is well documented that HIV increases the risk of TB infection and vice versa [
42,
43]; the current understanding of the human immune response to malaria, HIV, and TB make one to expect that TB infections could influence the clinical outcome of malaria in HIV clients. Infection with TB also weakens the body immune system increasing vulnerability to malaria infection.
The World Health Organization (WHO) recommends HIV management with combination antiretroviral therapy (ARV) [
44], All the patients were using ARV combination that consists of two nucleoside reverse-transcriptase inhibitors (NRTIs) plus a non-nucleoside reverse-transcriptase inhibitor (NNRTI) or an integrase inhibitor (INSTI). The proportion of subjects that has malaria parasitaemia was higher among those using AZT and lower among those using DGT.
This study also showed that rural dwellers had the highest proportion of those with HIV-malaria co-infection. This could be attributed to lack or inadequate materials for malaria prevention, such as insecticide treated nets, in the rural areas compared to urban areas [
45], leading to much exposure of rural dwellers to consistent bite by mosquitoes and therefore malaria. This is supported by the earlier report of Bassiouny and Al-Maktari in 2005 that rural areas have environmental conditions more favorable to transmission of the disease than urban areas [
46].
In our study also, poor knowledge of malaria infection and poor practice of malaria prevention were significant risk factors to malaria co-infection in HIV patients. In a systematic review of knowledge, attitudes and beliefs about malaria among the South Asian population, Krishna et al, shows that various measures to prevent malaria may exist but the success of these measures depends on the knowledge of, access to and utilization of services, as well as a combination of users´ behaviors and healthcare access and quality issues [
47]. This could help in explaining the concurrent findings of high knowledge of the subjects and high prevalence of malaria co-infection among the respondents in this study because it is not all knowledge that will translate to good practice.
Conclusion


From findings of this study, the prevalence of malaria co-infection among HIV patients in Zaria, Kaduna State is of public health concern. Having high HIV viral load, being co-infected with TB, having poor knowledge of malaria infection and poor practice of malaria infection prevention are significant risk factors for HIV-malaria co-infection. While place of residence, education level and occupation may modify or have a confounding effect on the association of these risk factors with the co-infection rate.
We therefore recommend that, because of their vulnerability to malaria, people living with HIV/AIDS should be considered among priority group for any malaria intervention; government at federal, state and local government levels should consider improving education of people as a means of tackling the co-infection; programmes for control of the two diseases and that of TB should collaborate to ensure integrated service delivery; these programmes should consider rural dwellers among priority groups; and additional research on interactions between the two diseases should be prioritized.
What is known about this topic
- HIV and malaria are endemic in Nigeria
- Kaduna state is among the states with high prevalence of HIV in Nigeria
What this study adds
- The prevalence of malaria co-infection among HIV patients in Zaria was high (22.9%)
- High HIV viral load, being co-infected with TB, poor knowledge of malaria infection, and poor practice of malaria prevention were risk factors for HIV-malaria co-infection
Competing interests


The authors declare that they have no competing interests
Authors' contributions


Shafi´u Dahiru Gumel, Abdulrasul Ibrahim, Adebola Tolulope Olayinka, Muhammed Sani Ibrahim, Muhammad Shakir Balogun, Ikeoluwapo Ajayi, and Olufemi Ajumobi contributed equally to this work and are co first authors.
AI,ATO. and MSI,MSB. conceived of the presented idea. MSI,MSB and IA, OA assisted in the development of the theory and in performing the computations. AI,ATO verified the analytical methods and supervised data management. IA,OA contributed in the development of data collection tools. AD, IA, AS and AIM,HA collected the data. MSI,MSB supported AI, ATO to investigate risk factors for HIV-Malaria co-infection and supervised the findings of this work.
IA.AS and AIM, HA. carried out the experiment. MSB, IA performed the analytic calculations and numerical simulations. MSI, MSB contributed to the interpretation of the results with support of all authors. AI, ATO took the lead in writing the manuscript. All authors provided critical feedback and helped shape the research, analysis and manuscript.
Acknowledgments


The authors wish to appreciate the staff of National TB Reference laboratory Saye-Zaria, who provided the much-needed support and a conducive atmosphere for the successful conduct of this study.
Tables and figures


Table 1: Socio-economic and demographic Risk Factors of HIV-Malaria co-, NTBLTC Zaria, 2018
Table 2: Clinical Risk Factors of HIV-Malaria co-infection, NTBLTC Zaria, 2018
Table 3: Factors Independently Associated with HIV-Malaria co-infection, NTBLTC Zaria, 2018
Table 4: Comparison of some immunological parameters between malaria positive and malaria negative, NTBLTC Zaria - 2018
Figure 1: Proportion of subjects with malaria parasitaemia by different ARV used, NTBLTC Zaria, 2018
Figure 2: Distribution of subjects by ARV combinations and malaria status, NTBLTC Zaria, 2018
Figure 3: Correlation of malaria parasite density with viral load of HIV subjects, NTBLTC Zaria, 2018
Figure 4: Correlation of malaria parasite density with CD4 count of HIV subjects, NTBLTC Zaria, 2018
References


- What are HIV and AIDS? [Internet]. Avert. Avert; 2015 [cited 2021 Feb 26]. https://www.avert.org/about-hiv-aids/what-hiv-aids. PubMed | Google Scholar
- National Malaria Elimination Programme (NMEP), National Population Commission (NPopC), National Bureau of Statistics (NBS), ICF International. Nigeria Malaria Indicator Survey 2015 [Internet]. Abuja, Nigeria, and Rockville, Maryland, USA; 2016 [cited 2021 Feb 27].. PubMed | Google Scholar
- Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009 Feb;60(1):471-84.https://doi.org/10.1146/annurev.med.60.041807.123549. PubMed | Google Scholar
- WHO | Malaria in HIV/AIDS patients[Internet]. WHO. [cited 2021 Feb 26].. PubMed | Google Scholar
- Aboud S, Msamanga GI, Mehta S, Fawzi WW, Franke MF, Spiegelman D, Ezeamama A. Malaria parasitemia and cd4 t cell count, viral load, and adverse HIV outcomes among HIV-infected pregnant women in Tanzania. The American Journal of Tropical Medicine and Hygiene. 2010 Apr 1;82(4):556-62.https://doi.org/10.4269/ajtmh.2010.09-0477. PubMed | Google Scholar
- Uju M. E. Dibua, Lorina Badger-Emeka, Joseph A. Ugonabo. HIV and Malaria co-infection: Their combined effects on pregnancy outcomes in Anambra State, Southeast Nigeria. Int J Med Med Sci. 2013 Oct;5(10):438-49.https://doi.org/10.5897/IJMMS2013.0903. PubMed | Google Scholar
- Malaria [Internet]. [cited 2021 Feb 26].. PubMed | Google Scholar
- WHO |Malaria in children under five [Internet]. WHO. [cited 2021 Feb 26].. PubMed | Google Scholar
- WHO | High-risk groups [Internet]. WHO. [cited 2021 Feb 26].. PubMed | Google Scholar
- Bartoloni A, Zammarchi L. Clinical aspects of uncomplicated and severe malaria. Mediterr J Hematol Infect Dis. 2012 May 4;4(1):e2012026.https://doi.org/4084/mjhid.2012.026. PubMed | Google Scholar
- Sanyaolu AO, Fagbenro-Beyioku AF, Oyibo WA, Badaru OS, Onyeabor OS, Nnaemeka CI. Malaria and HIV co-infection and their effect on haemoglobin levels from three healthcare institutions in Lagos, southwest Nigeria. African Health Sciences. 2013 Sep 2;13(2):295-300.https://dx.doi.org/10.4314%2Fahs.v13i2.14. PubMed | Google Scholar
- UNAIDS. 2017 Global HIV Statistics: Fact Sheet-July 2018 [Internet]. UNAIDS; 2018 [cited 2021 Feb 27].. PubMed | Google Scholar
- Global HIV and AIDS Statistics [Internet]. Avert; 2018 [cited 2021 Feb 27].. PubMed | Google Scholar
- World Health Organization, Global Malaria Programme. World Malaria Report 2018 [Internet]. Geneva: World Health Organization; 2018 [cited 2021 Feb 27].. PubMed | Google Scholar
- World Health Organization. World Malaria Report 20 [Internet]. World Health Organization; 2016 [cited 2021 Feb 26].. PubMed | Google Scholar
- Idemyor V. Review: Human Immunodeficiency Virus (HIV) and Malaria interaction in Sub-Saharan Africa: the collision of two titans. HIV Clinical Trials. 2007 Aug;8(4):246-53. https://doi.org/10.1310/hct0804-246. PubMed | Google Scholar
- Kwenti TE. Malaria and HIV coinfection in sub-Saharan Africa: prevalence, impact, and treatment strategies [Internet]. Research and Reports in Tropical Medicine. 2018 [cited 2021 Feb 26].Available from: https://www.dovepress.com/malaria-and-hiv-coinfection-in-sub-saharan-africa-prevalence-impact-an-peer-reviewed-article-RRTM https://dx.doi.org/10.2147%2FRRTM.S154501. PubMed | Google Scholar
- Tay SC, Badu K, Mensah AA, Gbedema SY. The prevalence of malaria among HIV seropositive individuals and the impact of the co- infection on their hemoglobin levels. Annals of Clinical Microbiology and Antimicrobials. 2015 Mar 7;14(1):10.https://dx.doi.org/10.1186%2Fs12941-015-0064-6. PubMed | Google Scholar
- World Health Organization, World Health Organization, Department of HIV/AIDS, Global Partnership to Roll Back Malaria, editors. Malaria and HIV interactions and their implications for public health policy. [Internet]. Geneva: World Health Organization; 2005 [cited 2021 Feb 27].. PubMed | Google Scholar
- Kamya MR, Gasasira AF, Yeka A, Bakyaita N, Nsobya SL, Francis D, Rosenthal PJ, Dorsey G, Havlir D. Effect of hiv?1 infection on antimalarial treatment outcomes in uganda: a population?based study. J Infect Dis. 2006 Jan;193(1):9-15. https://doi.org/10.1086/498577. PubMed | Google Scholar
- Brentlinger PE. Challenges in the prevention, diagnosis, and treatment of Malaria in Human Immunodeficiency Virus-infected adults in sub-saharan africa. Arch Intern Med. 2007 Sep 24;167(17):1827. https://doi.org/10.1001/archinte.167.17.1827. PubMed | Google Scholar
- Hobbs CV, Neal J, Conteh S, Donnelly L, Chen J, Marsh K, Lambert L, Orr-Gonzalez S, Hinderer J, Healy S, Borkowsky W, Penzak SR, Chakravarty S, Hoffman SL, Duffy PE. HIV treatments reduce Malaria liver stage burden in a non-human primate model of Malaria infection at clinically relevant concentrations in vivo. Russell B, editor. PLoS ONE. 2014 Jul 2;9(7):e100138.https://doi.org/10.1371/journal.pone.0100138. PubMed | Google Scholar
- Alfonso Y, Monzote L. HIV protease inhibitors: effect on the opportunistic protozoan parasites. Open Med Chem J. 2011 Mar 9;5:40-50.https://doi.org/10.2174/1874104501105010040. PubMed | Google Scholar
- National Malaria Elimination Programme, Federal Ministry of Health, Abuja, Nigeria. National Malaria Strategic Plan 2014-2020 [Internet]. Federal Ministry of Health, Abuja, Nigeria. [cited 2021 Feb 27].. PubMed | Google Scholar
- Guidelines for the treatment of malaria[Internet]. Third. Geneva: World Health Organization; 2015 [cited 2021 Feb 26]. 313 p.. PubMed | Google Scholar
- Federal Ministry of Health, Nigeria. National HIV&AIDS and Reproductive Health Survey (NARHS Plus II, 2012) [Internet]. Abuja, Nigeria: Federal Ministry of Health Abuja, Nigeria; 2013 Nov [cited 2021 Feb 26].. PubMed | Google Scholar
- Akinyemi Gabriel Omonijo. Rainfall Amount and Number of Raindays in Kaduna, Northern Nigeria-Implication on Crop Production. In: International Conference on Agricultural, Ecological and Medical Sciences (AEMS-2014) [Internet]. London (United Kingdom): International Institute of Chemical, Biological & Environmental Engineering (IICBEE); 2014 [cited 2021 Feb 26]. p. 7. Available from: https://iicbe.org/siteadmin/upload/5323C714048.pdf http://dx.doi.org/10.15242/IICBE.C714. PubMed | Google Scholar
- Lwanga SK, Lemeshow S. Sample size determination in health studies: a practical manual [Internet]. Geneva: World Health Organization; 1991 [cited 2021 Feb 27]. 80 p.. PubMed | Google Scholar
- Uneke CJ, Ogbu O, Inyama PU, Anyanwu GI. Malaria infection in HIV-seropositive and HIV-seronegative individuals in Jos-Nigeria. J Vector Borne Dis. 2005 Dec;42(4):151-4.. PubMed | Google Scholar
- Taiwan CDC Free Anonymous HIV Testing and Counseling Consent Form [Internet]. Taiwan Centers of Disease Control ; 2016 [cited 2021 Feb 27].. PubMed | Google Scholar
- Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC), National Bureau of Statistics (NBS), Office of the Chief Government Statistician (OCGS), ICF International. Tanzania HIV/AIDS and Malaria Indicator Survey 2011-12 [Internet]. Dar es Salaam, Tanzania; 2013 [cited 2021 Feb 27].. PubMed | Google Scholar
- HIV testing, treatment and prevention: generic tools for operational research: Client Questionnaire[Internet]. World Health Organization; 2009 [cited 2021 Feb 27].. PubMed | Google Scholar
- Malaria Indicator Survey Sample Household Questionnaire [Internet]. World Health Organization; 2020 [cited 2021 Feb 27].. PubMed | Google Scholar
- McMahon JH, Elliott JH, Bertagnolio S, Kubiak R, Jordan MR. Viral suppression after 12 months of antiretroviral therapy in low- and middle-income countries: a systematic review. Bull World Health Organ. 2013 May 1;91(5):377-385E.https://dx.doi.org/10.2471/BLT.12.112946. PubMed | Google Scholar
- WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. [Internet]. World Health Organization; 2016 [cited 2021 Feb 26].. PubMed | Google Scholar
- Cheesbrough M. District laboratory practice in tropical countries. Part 1. Second. Cambridge University Press; 2010.https://doi.org/10.1017/CBO9780511581304. PubMed | Google Scholar
- Malaria Parasite Counting: Malaria Microscopy Standard Operating Procedure- MM-SOP-09 [Internet]. World Health Organization; 2016 [cited 2021 Feb 27].. PubMed | Google Scholar
- Onyenekwe CC, Ukibe N, Meludu SC, Ilika A, Aboh N, Ofiaeli N, Ezaeni M, Onochie A. Prevalence of malaria as co-infection in HIV-infected individuals in a malaria endemic area of southeastern Nigeria. J Vector Borne Dis. 2007 Dec;44(4):250-4.. PubMed | Google Scholar
- Hasang W, Dembo EG, Wijesinghe R, Molyneux ME, Kublin JG, Rogerson S. HIV-1 infection and antibodies to plasmodium falciparum in adults. Journal of Infectious Diseases. 2014 Nov 1;210(9):1407-14.https://doi.org/10.1093/infdis/jiu262. PubMed | Google Scholar
- Jegede FE, Oyeyi TI, Abdulrahman SA, Mbah HAkwen, Badru T, Agbakwuru C, Adedokun O. Effect of HIV and malaria parasites co-infection on immune-hematological profiles among patients attending anti-retroviral treatment (Art) clinic in Infectious Disease Hospital Kano, Nigeria. Arez AP, editor. PLoS ONE. 2017 Mar 27;12(3):e0174233.https://doi.org/10.1371/journal.pone.0174233. PubMed | Google Scholar
- Chaisavaneeyakorn S, Moore JM, Mirel L, Othoro C, Otieno J, Chaiyaroj SC, Shi YP, Nahlen BL, Lal AA, Udhayakumar V. Levels of macrophage inflammatory protein 1? (MIP-1?) and mip-1? in intervillous blood plasma samples from women with placental malaria and human immunodeficiency virus infection. CVI. 2003 Jul;10(4):631-6.https://doi.org/10.1128/cdli.10.4.631-636.2003. PubMed | Google Scholar
- TB & HIV coinfection | Basic TB facts | TB | CDC [Internet]. 2020 [cited 2021 Feb 26].. PubMed | Google Scholar
- Pawlowski A, Jansson M, Sk�ld M, Rottenberg ME, K�llenius G. Tuberculosis and hiv co-infection. PLOS Pathogens. 2012 Feb 16;8(2):e1002464.https://doi.org/10.1371/journal.ppat.1002464. PubMed | Google Scholar
- WHO. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing hiv infection: recommendations for a public health approach. [Internet]. Second. Geneva: World Health Organization; 2016 [cited 2021 Feb 26]. 480 p.https://www.who.int/hiv/pub/arv/arv-2016/en/. PubMed | Google Scholar
- Ndwiga T, Kei RM, Dancan OW. Utilization of insecticide treated bed nets among mothers attending MCH/FP in Webuye District Hospital, Bungoma county, Kenya. Open Journal of Preventive Medicine. 2014 Jun 11;4(6):470-80. http://dx.doi.org/10.4236/ojpm.2014.46055. PubMed | Google Scholar
- Bassiouny HK, Al Maktari MT. Malaria in late pregnancy in Al Hodeidah governorate, Yemen. EMHJ - Eastern Mediterranean Health Journal, 11 (4), 606-617, 2005 [Internet]. 2005 [cited 2021 Feb 26]; https://apps.who.int/iris/handle/10665/116985. PubMed | Google Scholar
- Regmi K, Kunwar A, Ortega L. A systematic review of knowledge, attitudes and beliefs about malaria among the South Asian population. Infection Ecology & Epidemiology. 2016 Jan 1;6(1):30822. https://dx.doi.org/10.3402%2Fiee.v6.30822. PubMed | Google Scholar
>